- +86 185 8909 5435
- sales@senovaph.com
Your Location:Home >Products >761440-16-8

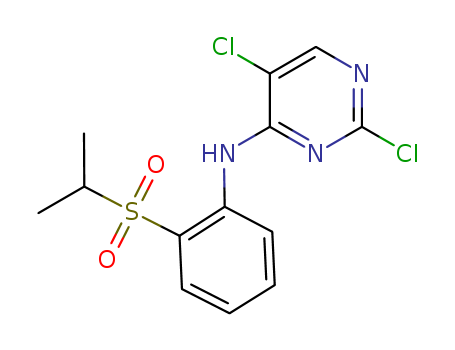
Purity:99%
|
Uses |
2,5-Dichloro-N-[2-(isopropylsulfonyl)phenyl]pyrimidin-4-amine is used as a reagent in the synthesis of potent and selective anaplastic lymphoma kinase (ALK-5) inhibitors, used as an anti-tumor treatment. |
InChI:InChI=1S/C13H13Cl2N3O2S/c1-8(2)21(19,20)11-6-4-3-5-10(11)17-12-9(14)7-16-13(15)18-12/h3-8H,1-2H3,(H,16,17,18)
Aiming to identify novel potent ALK and ...
The present invention provides a small m...
Disclosed are bispecific compounds (degr...
The invention provides a CDK inhibitor b...
PROBLEM TO BE SOLVED: To provide a proce...
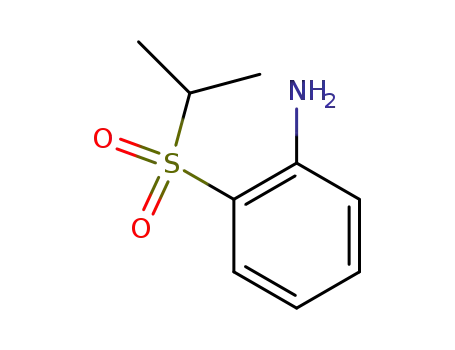
1-amino-2-(isopropylsulphonyl)benzene

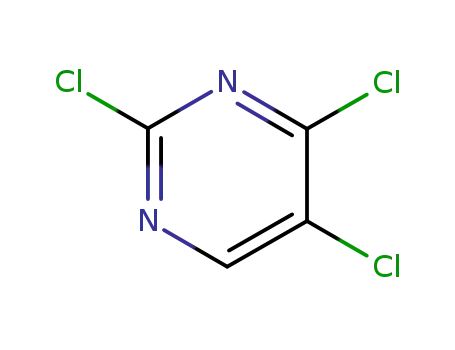
2,4,5-trichloropyrimidine

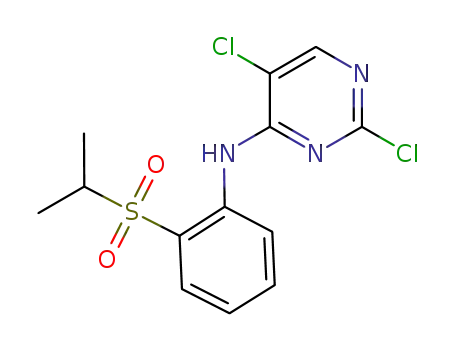
2-chloro-N-(2-(isopropylsulfonyl)phenyl)-5-methyl-pyrimidin-4-amine
| Conditions | Yield |
|---|---|
|
With potassium tert-butylate; zinc diacetate; In 1-methyl-pyrrolidin-2-one; at 118 - 122 ℃; for 10h; Reagent/catalyst; Temperature; Solvent;
|
92.1% |
|
1-amino-2-(isopropylsulphonyl)benzene; With sodium hydride; In dimethyl sulfoxide; N,N-dimethyl-formamide; at 0 ℃; for 0.5h; Inert atmosphere;
2,4,5-trichloropyrimidine; In dimethyl sulfoxide; N,N-dimethyl-formamide; at 0 - 20 ℃; Inert atmosphere;
|
90.6% |
|
2,4,5-trichloropyrimidine; With sodium hydride; In N,N-dimethyl-formamide; mineral oil; at 0 ℃; for 0.5h;
1-amino-2-(isopropylsulphonyl)benzene; In N,N-dimethyl-formamide; mineral oil; at 0 - 20 ℃; for 2h;
|
72% |
|
With palladium diacetate; caesium carbonate; triphenylphosphine; In toluene; for 4h; Inert atmosphere; Reflux;
|
65.6% |
|
1-amino-2-(isopropylsulphonyl)benzene; With sodium hydride; In N,N-dimethyl-formamide; mineral oil; at 0 ℃; for 1h; Ionic liquid;
2,4,5-trichloropyrimidine; In N,N-dimethyl-formamide; mineral oil; at 20 ℃;
|
63% |
|
1-amino-2-(isopropylsulphonyl)benzene; With sodium hydride; In dimethyl sulfoxide; N,N-dimethyl-formamide; at 0 ℃;
2,4,5-trichloropyrimidine; In dimethyl sulfoxide; N,N-dimethyl-formamide; at 0 - 20 ℃;
|
60% |
|
1-amino-2-(isopropylsulphonyl)benzene; With sodium hydride; In dimethyl sulfoxide; N,N-dimethyl-formamide; at 0 ℃; for 0.75h; Inert atmosphere;
2,4,5-trichloropyrimidine; In dimethyl sulfoxide; N,N-dimethyl-formamide; at 0 - 20 ℃; for 2.75h;
|
60.6% |
|
1-amino-2-(isopropylsulphonyl)benzene; With sodium hydride; In N,N-dimethyl-formamide; at 0 ℃; for 0.5h;
2,4,5-trichloropyrimidine; In N,N-dimethyl-formamide; at 0 - 20 ℃; regioselective reaction;
|
59% |
|
With sodium hydride; In dimethyl sulfoxide; N,N-dimethyl-formamide; mineral oil; at 20 ℃; for 16h; Concentration; Solvent; Cooling with ice;
|
52% |
|
1-amino-2-(isopropylsulphonyl)benzene; With sodium hydride; In N,N-dimethyl-formamide; mineral oil; at 0 ℃; for 0.5h;
2,4,5-trichloropyrimidine; In N,N-dimethyl-formamide; mineral oil; at 20 ℃;
|
48% |
|
With sodium hydride; In N,N-dimethyl-formamide; mineral oil; at 25 ℃; for 12h; Temperature; Solvent;
|
46% |
|
With sodium hydride; In N,N-dimethyl-formamide; mineral oil; at 25 ℃; for 12h;
|
46% |
|
1-amino-2-(isopropylsulphonyl)benzene; With sodium hydride; In dimethyl sulfoxide; N,N-dimethyl-formamide; at 0 ℃; for 0.5h;
2,4,5-trichloropyrimidine; In dimethyl sulfoxide; N,N-dimethyl-formamide; at 0 - 20 ℃; for 15h;
|
40.6% |
|
1-amino-2-(isopropylsulphonyl)benzene; With sodium hydride; In N,N-dimethyl-formamide; at 0 ℃; for 0.5h;
2,4,5-trichloropyrimidine; In N,N-dimethyl-formamide; at 20 ℃; for 12h;
|
40% |
|
1-amino-2-(isopropylsulphonyl)benzene; With sodium hydride; In N,N-dimethyl-formamide; mineral oil; for 0.5h; Cooling with ice;
2,4,5-trichloropyrimidine; In N,N-dimethyl-formamide; mineral oil; for 10h; Cooling with ice;
|
40% |
|
1-amino-2-(isopropylsulphonyl)benzene; With sodium hydride; In N,N-dimethyl-formamide; mineral oil; for 0.5h; Cooling with ice;
2,4,5-trichloropyrimidine; In N,N-dimethyl-formamide; for 10h; Cooling with ice;
|
40% |
|
1-amino-2-(isopropylsulphonyl)benzene; With sodium hydride; In N,N-dimethyl-formamide; for 0.5h; Cooling with ice;
2,4,5-trichloropyrimidine; In N,N-dimethyl-formamide; at 20 ℃; Cooling with ice;
|
40% |
|
1-amino-2-(isopropylsulphonyl)benzene; With sodium hydride; In N,N-dimethyl-formamide; at 0 ℃; for 0.666667h;
2,4,5-trichloropyrimidine; In N,N-dimethyl-formamide; at 0 - 20 ℃;
|
35% |
|
1-amino-2-(isopropylsulphonyl)benzene; With sodium hydride; In N,N-dimethyl-formamide; at 0 ℃; for 0.25h;
2,4,5-trichloropyrimidine; In N,N-dimethyl-formamide; at 20 ℃;
|
33% |
|
1-amino-2-(isopropylsulphonyl)benzene; With sodium hydride; In N,N-dimethyl-formamide; for 0.25h; Cooling;
2,4,5-trichloropyrimidine; In N,N-dimethyl-formamide; at 20 ℃; for 16h;
|
32.69% |
|
1-amino-2-(isopropylsulphonyl)benzene; With sodium hydride; In N,N-dimethyl-formamide; at 0 ℃; for 0.25h;
2,4,5-trichloropyrimidine; In N,N-dimethyl-formamide; at 20 ℃;
|
32% |
|
1-amino-2-(isopropylsulphonyl)benzene; With sodium hydride; In N,N-dimethyl-formamide; at 0 - 5 ℃; for 0.25h;
2,4,5-trichloropyrimidine; In N,N-dimethyl-formamide; at 0 - 5 ℃;
|
32.7% |
|
1-amino-2-(isopropylsulphonyl)benzene; With sodium hydride; In N,N-dimethyl-formamide; mineral oil; at 0 ℃; for 0.333333h;
2,4,5-trichloropyrimidine; In N,N-dimethyl-formamide; mineral oil; at 0 - 20 ℃; for 2.5h;
|
20% |
|
With N-ethyl-N,N-diisopropylamine; In isopropyl alcohol; for 2h; Reflux;
|
17% |
|
1-amino-2-(isopropylsulphonyl)benzene; With sodium hydride; In N,N-dimethyl-formamide; mineral oil; at 0 ℃; for 0.5h;
2,4,5-trichloropyrimidine; In N,N-dimethyl-formamide; mineral oil; at 0 - 20 ℃; for 12h;
|
|
|
With sodium hydride; In N,N-dimethyl-formamide;
|
|
|
1-amino-2-(isopropylsulphonyl)benzene; With sodium hydride; In N,N-dimethyl-formamide; mineral oil; at 0 ℃; for 4h; Inert atmosphere;
2,4,5-trichloropyrimidine; In N,N-dimethyl-formamide; mineral oil; at 0 ℃; for 4h; Inert atmosphere;
|
|
|
With potassium carbonate; In N,N-dimethyl-formamide; at 75 ℃;
|
|
|
1-amino-2-(isopropylsulphonyl)benzene; With sodium hydride; In N,N-dimethyl acetamide; at 0 ℃; for 1h;
2,4,5-trichloropyrimidine; In N,N-dimethyl acetamide; at 20 - 25 ℃; for 2h; Reagent/catalyst; Time; Temperature;
|
9.1 g |
|
1-amino-2-(isopropylsulphonyl)benzene; With sodium hydride; In N,N-dimethyl-formamide; at 20 ℃; for 0.5h; Cooling with ice;
2,4,5-trichloropyrimidine; In N,N-dimethyl-formamide; at 20 ℃; for 16h;
|
1.7 g |
|
With 1,8-diazabicyclo[5.4.0]undec-7-ene; at 80 ℃; for 7.5h;
|
|
|
With sodium hydride; In N,N-dimethyl-formamide; at 20 ℃;
|
0.5 g |
|
With 1,8-diazabicyclo[5.4.0]undec-7-ene; In tetrahydrofuran; at 80 ℃; Solvent; Temperature; Reagent/catalyst; Inert atmosphere;
|
0.88 g |
|
With sodium hydride; In dimethyl sulfoxide;
|
|
|
1-amino-2-(isopropylsulphonyl)benzene; With sodium hydride; In N,N-dimethyl-formamide; at -10 ℃; for 0.5h;
2,4,5-trichloropyrimidine; In N,N-dimethyl-formamide; at 20 ℃; for 6h;
|
|
|
With 1,8-diazabicyclo[5.4.0]undec-7-ene; at 80 ℃; for 7.5h;
|
|
|
With 1,8-diazabicyclo[5.4.0]undec-7-ene; at 80 ℃; for 7.5h; Large scale;
|
1.07 kg |
|
1-amino-2-(isopropylsulphonyl)benzene; With sodium hydride; In dimethyl sulfoxide; N,N-dimethyl-formamide; at 0 ℃; for 0.5h;
2,4,5-trichloropyrimidine;
|
|
|
1-amino-2-(isopropylsulphonyl)benzene; With sodium hydride; In N,N-dimethyl-formamide; at 0 ℃; for 0.5h;
2,4,5-trichloropyrimidine; In N,N-dimethyl-formamide; at 20 ℃; for 2h;
|
2.7 g |
|
1-amino-2-(isopropylsulphonyl)benzene; With sodium hydride; In N,N-dimethyl-formamide; at 0 ℃; for 0.5h;
2,4,5-trichloropyrimidine; In N,N-dimethyl-formamide; at 20 ℃; for 2h;
|
2.7 g |
|
With 1,8-diazabicyclo[5.4.0]undec-7-ene; at 80 ℃; for 7.5h; Large scale;
|
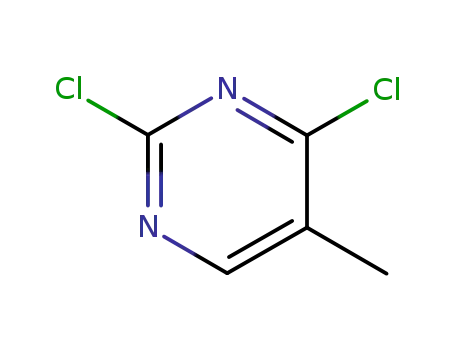
2,6-dichloro-5-methylpyrimidine


1-amino-2-(isopropylsulphonyl)benzene


2-chloro-N-(2-(isopropylsulfonyl)phenyl)-5-methyl-pyrimidin-4-amine
| Conditions | Yield |
|---|---|
|
1-amino-2-(isopropylsulphonyl)benzene; With sodium hydride; In N,N-dimethyl-formamide; at 0 ℃; for 0.5h;
2,6-dichloro-5-methylpyrimidine; In N,N-dimethyl-formamide; at 20 ℃;
|
59% |
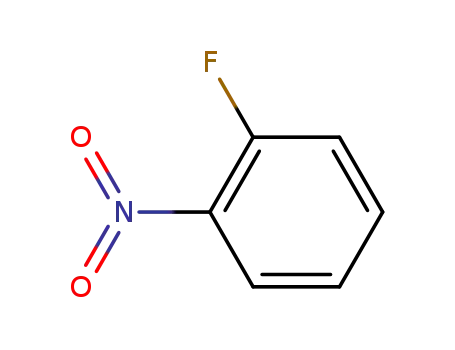
ortho-nitrofluorobenzene

1-amino-2-(isopropylsulphonyl)benzene

2,4,5-trichloropyrimidine
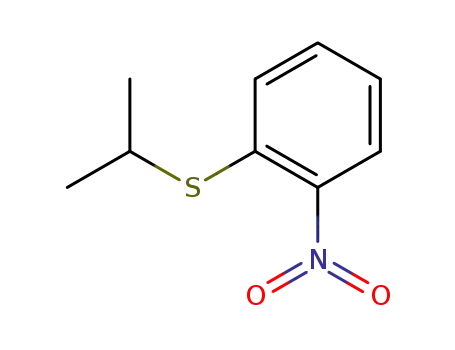
2-(isopropyl sulfanyl)nitrobenzene
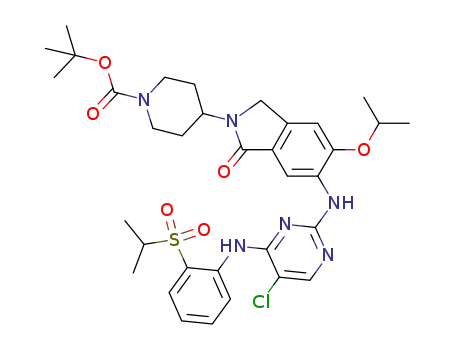
C34H43ClN6O6S
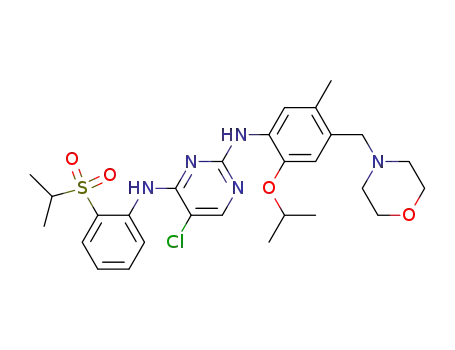
5-chloro-N2-(2-isopropoxy-5-methyl-4-morpholin-4-ylmethyl-phenyl)-N4-[2-(propane-2-sulfonyl)-phenyl]-pyrimidine-2,4-diamine
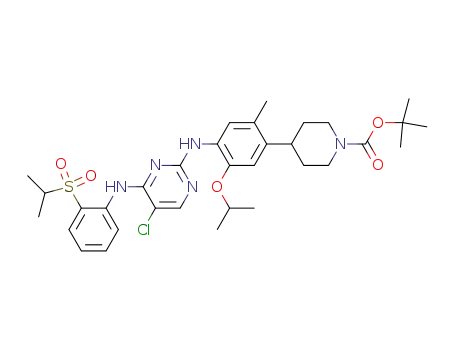
4-(4-{5-chloro-4-[2-(propane-2-sulfonyl)phenylamino]pyrimidin-2-ylamino}-5-isopropoxy-2-methylphenyl)piperidine-1-carboxylic acid tert-butyl ester
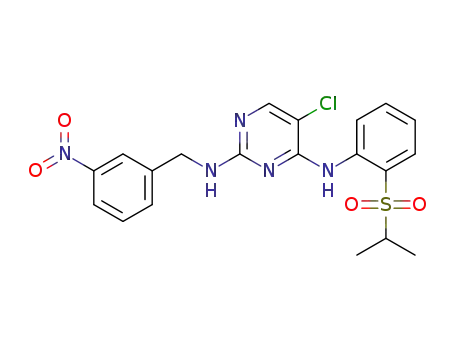
5-chloro-N4-(2-(isopropylsulfonyl)phenyl)-N2-(3-nitrobenzyl)pyrimidine-2,4-diamine