- +86 185 8909 5435
- sales@senovaph.com
Your Location:Home >Products >Pharmaceutical intermediates >314771-76-1

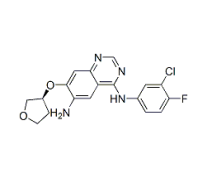
Purity:99%
|
Uses |
(S)-N4-(3-chloro-4-fluorophenyl)-7-(tetrahydrofuran-3-yloxy)quinazoline-4,6-diamine can be used as organic synthesis intermediate and pharmaceutical intermediate, mainly used in laboratory research and development process and chemical production process. |
|
Synthesis |
To a solution of 4-(3-chloro-4-fluorophenyl)amino-6-nitro-7-[[(S)-tetrahydro-3-furanyl]oxy]- quinazoline (1.34 g, 3.31 mmol) and ammonium chloride (496 mg, 9.27 mmol) in anhydrous DMF (22 mL), Raney nickel [1.5 mL, 50 % (w/v) in water] was added to the reaction mixture and stirred under an atmosphere of hydrogen at 40 °C for 2 h. After 2 h the reaction was diluted with EtOH (10 mL), filtered through diatomaceous earth and washed with a large excess of EtOH. The residue was concentrated under reduced pressure and purified by column chromatography eluting with CHCl3/MeOH (95:5) to give 6 as a viscous brown oil (950 mg, 77 %). |
Afatinib is an irreversible tyrosine kin...
The development of green and efficient m...
The invention discloses an afatinib inte...
The invention discloses a synthesis meth...
The invention discloses a preparation me...
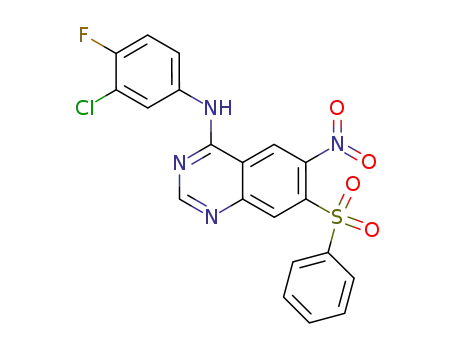
N-((3-chloro-4-fluorophenyl)-6-nitro-7-phenylsulfonyl)quinazolin-4-one

![6-Amino-4-[(3-chloro-4-fluorophenyl)amino]-7-[(S)-(tetrahydrofuran-3-yl)oxy]quinazoline](/upload/2023/5\189558d0-2efc-436f-aad8-be9ffe99c766.png)
6-Amino-4-[(3-chloro-4-fluorophenyl)amino]-7-[(S)-(tetrahydrofuran-3-yl)oxy]quinazoline
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: sodium hydride / N,N-dimethyl-formamide / 4 h / 0 - 20 °C
2: hydrogen / N,N-dimethyl-formamide / 4 h
With hydrogen; sodium hydride; In N,N-dimethyl-formamide;
|
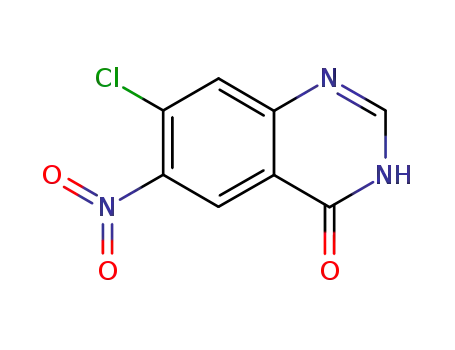
7-chloro-6-nitro-4(3H)-quinazolinone

![6-Amino-4-[(3-chloro-4-fluorophenyl)amino]-7-[(S)-(tetrahydrofuran-3-yl)oxy]quinazoline](/upload/2023/5\189558d0-2efc-436f-aad8-be9ffe99c766.png)
6-Amino-4-[(3-chloro-4-fluorophenyl)amino]-7-[(S)-(tetrahydrofuran-3-yl)oxy]quinazoline
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 5 steps
1: trichlorophosphate / 2 h / 90 °C
2: 1,4-dioxane / 3 h / 90 °C
3: 2 h / 90 °C
4: sodium hydride / N,N-dimethyl-formamide / 4 h / 0 - 20 °C
5: hydrogen / N,N-dimethyl-formamide / 4 h
With hydrogen; sodium hydride; trichlorophosphate; In 1,4-dioxane; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 3 steps
1.1: thionyl chloride / N,N-dimethyl-formamide / 3 h / Reflux
1.2: 1 h / 90 °C
2.1: potassium tert-butylate / N,N-dimethyl-formamide / 2 h / 0 °C
2.2: 6 h / 30 - 90 °C
3.1: iron(III) chloride hexahydrate; pyrographite; hydrazine hydrate / ethanol / 1 h / Reflux
With thionyl chloride; iron(III) chloride hexahydrate; potassium tert-butylate; pyrographite; hydrazine hydrate; In ethanol; N,N-dimethyl-formamide;
|
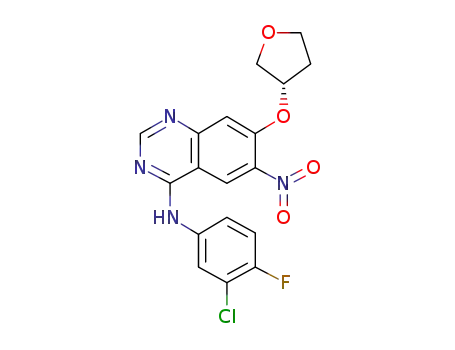
4-[(3-chloro-4-fluorophenyl)amino]-6-nitro-7-[(S)-(tetrahydrofuran-3-yl)oxy]quinazoline
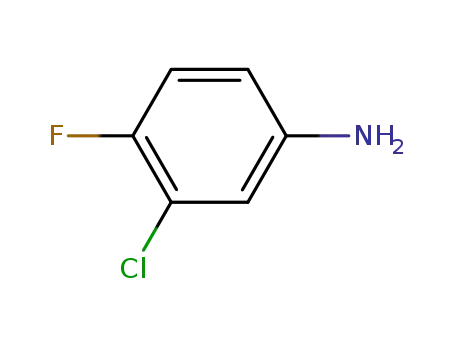
3-chloro-4-fluorophenylamine
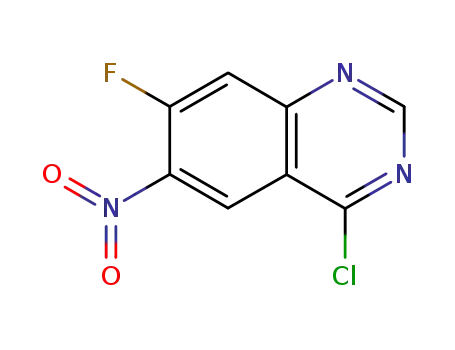
4-chloro-7-fluoro-6-nitroquinazoline
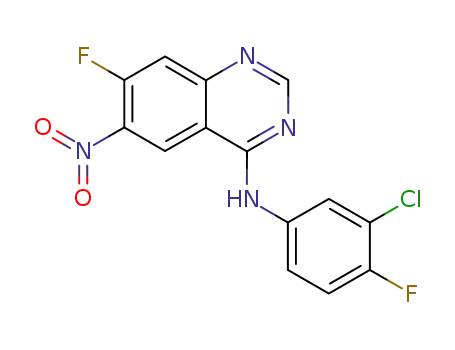
N-(3-chloro-4-fluorophenyl)-7-fluoro-6-nitroquinazolin-4-amine