- +86 185 8909 5435
- sales@senovaph.com
Your Location:Home >Products >Pharmaceutical intermediates >253870-02-9

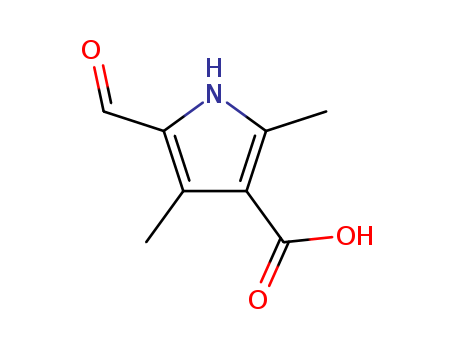
Purity:99%
|
Description |
5-Formyl-2,4-dimethyl-1H-pyrrole-3-carboxylic acid is an important organic intermediate to synthetize substituted pyrrole products. |
|
Uses |
5-Formyl-2,4-dimethyl-1H-pyrrole-3-carboxylic Acid is useful for the synthesis of 5-Bromo-7-azaindolin-2-one derivatives which possesses in vitro activity against selected cancer cell lines. |
InChI:InChI=1/C8H9NO3/c1-4-6(3-10)9-5(2)7(4)8(11)12/h3,9H,1-2H3,(H,11,12)
The presented study explores the antican...
A potential molecular hybridization stra...
The use of covalent irreversible binding...
The invention discloses an enhanced fluo...
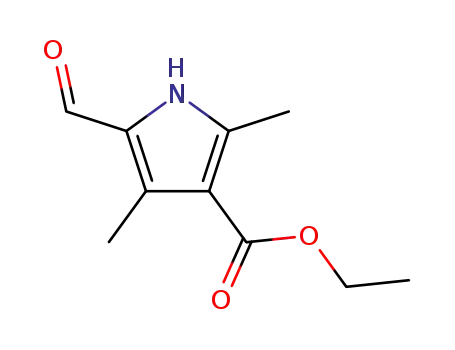
ethyl (5-formyl-2,4-dimethyl-1H-pyrrole)-3-carboxylate

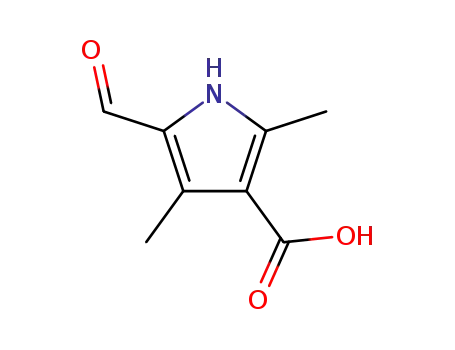
5-formyl-2,4-dimethyl-1H-pyrrole-3-carboxylic acid
| Conditions | Yield |
|---|---|
|
With potassium hydroxide; In methanol; water; Reflux;
|
99% |
|
With potassium hydroxide; In methanol; water; at 70 ℃;
|
97% |
|
With methanol; water; potassium hydroxide; at 100 ℃; for 2h;
|
94% |
|
With water; potassium hydroxide; In methanol; for 3h; Reflux;
|
94% |
|
ethyl (5-formyl-2,4-dimethyl-1H-pyrrole)-3-carboxylate; With potassium hydroxide; In methanol; water; for 3h;
With hydrogenchloride;
|
94% |
|
With potassium hydroxide; In methanol;
|
93% |
|
With potassium hydroxide; water; In methanol; for 3h; Heating / reflux;
|
93% |
|
ethyl (5-formyl-2,4-dimethyl-1H-pyrrole)-3-carboxylate; With potassium hydroxide; water; In methanol; for 3h; Heating / reflux;
With hydrogenchloride; In methanol; water; at 20 ℃; pH=3;
|
93% |
|
ethyl (5-formyl-2,4-dimethyl-1H-pyrrole)-3-carboxylate; With water; potassium hydroxide; In methanol; at 60 - 70 ℃; for 4 - 6h;
With hydrogenchloride; In methanol; water; at 25 - 30 ℃; pH=4.0;
|
93.5% |
|
With water; potassium hydroxide; In methanol; at 60 - 70 ℃; for 4 - 6h;
|
93.5% |
|
With water; potassium hydroxide; In methanol; for 4h; Reflux;
|
93% |
|
ethyl (5-formyl-2,4-dimethyl-1H-pyrrole)-3-carboxylate; With potassium hydroxide; water; In methanol; for 3h; Heating / reflux;
With hydrogenchloride; In methanol; water; pH=3;
|
93% |
|
With sodium hydroxide; In methanol; water; for 4h; Reflux;
|
92% |
|
With water; sodium hydroxide; In methanol; at 82 - 83 ℃; for 4.5h; Inert atmosphere;
|
91% |
|
With sodium hydroxide; In methanol; water; for 4.5h; Reflux; Inert atmosphere;
|
91% |
|
With potassium hydroxide; In methanol; for 3h; Heating;
|
90% |
|
ethyl (5-formyl-2,4-dimethyl-1H-pyrrole)-3-carboxylate; With potassium hydroxide; ethanol; water; Heating / reflux;
With hydrogenchloride; water; pH=2;
|
89% |
|
ethyl (5-formyl-2,4-dimethyl-1H-pyrrole)-3-carboxylate; With potassium hydroxide; water; In ethanol; Heating / reflux;
With hydrogenchloride; In ethanol; water; pH=2;
|
89% |
|
ethyl (5-formyl-2,4-dimethyl-1H-pyrrole)-3-carboxylate; With potassium hydroxide; In water; for 5h; Reflux;
With hydrogenchloride; In water; pH=4;
|
89% |
|
With potassium hydroxide; In methanol; water; for 4h; Reflux;
|
88% |
|
With potassium hydroxide; In methanol; water; for 3h; Reflux;
|
88% |
|
With water; potassium hydroxide; In methanol; at 80 ℃; for 3h;
|
86.3% |
|
ethyl (5-formyl-2,4-dimethyl-1H-pyrrole)-3-carboxylate; With water; potassium hydroxide; In ethanol; at 120 ℃; for 1.5h; Inert atmosphere;
With hydrogenchloride; In ethanol; water;
|
81% |
|
With potassium hydroxide; In methanol; water; for 4h; Reflux;
|
40% |
|
With sodium hydroxide;
|
|
|
With potassium hydroxide; In methanol; water;
|
1.6 g (93%) |
|
With potassium hydroxide; In methanol; water;
|
1.6 g (93%) |
|
ethyl (5-formyl-2,4-dimethyl-1H-pyrrole)-3-carboxylate; With water; potassium hydroxide; In methanol; at 75 - 85 ℃; for 6h;
With hydrogenchloride; In water; at 0 - 5 ℃; pH=4 - 5;
|
|
|
With potassium hydroxide; In methanol; Reflux;
|
1.14 g |
|
With potassium hydroxide; In water; for 4h; Reflux;
|
|
|
With potassium hydroxide; for 5h; Reflux;
|
1.14 g |
|
With water; potassium hydroxide; In methanol; for 3h; Reflux;
|
30 g |
|
With water; potassium hydroxide; In methanol; for 5h; Reflux;
|

ethyl (5-formyl-2,4-dimethyl-1H-pyrrole)-3-carboxylate


5-formyl-2,4-dimethyl-1H-pyrrole-3-carboxylic acid

5-[5-fluoro-2-oxo-1,2-dihydro-indol-(3Z)-ylidene-methyl]-2,4-dimethyl-1H-pyrrole-3-carboxylic Acid
| Conditions | Yield |
|---|---|
|
With potassium hydroxide; In methanol; water;
|
93% |
|
With potassium hydroxide; In methanol; water;
|
93% |
|
With potassium hydroxide; In methanol; water;
|
93% |

ethyl (5-formyl-2,4-dimethyl-1H-pyrrole)-3-carboxylate
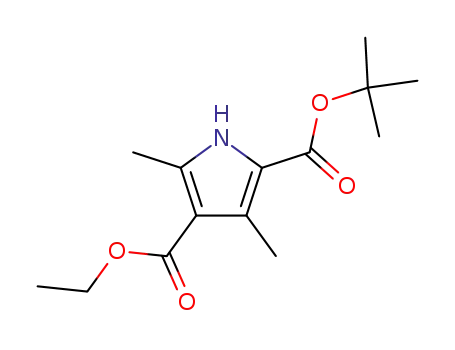
2-tert-butyl 4-ethyl 3,5-dimethyl-1H-pyrrole-2,4-dicarboxylate
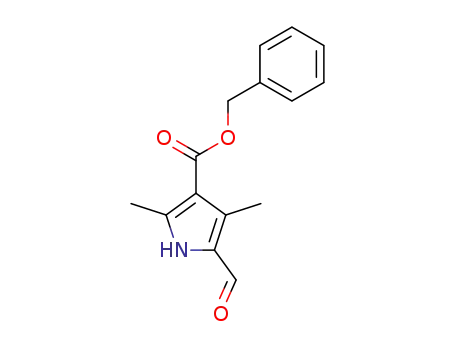
benzyl 5-formyl-2,4-dimethyl-1H-pyrrole-3-carboxylate
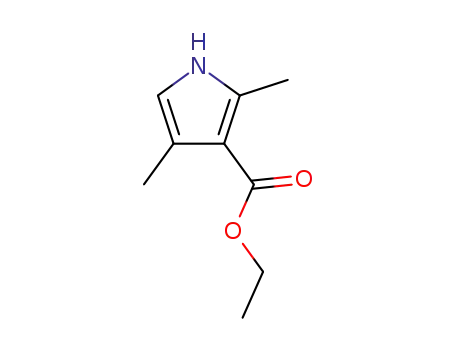
2,4-dimethyl-1H-pyrrole-3-carboxylic acid ethyl ester
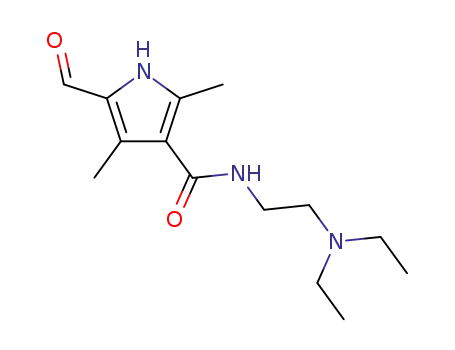
N-(2-(diethylamino)ethyl)-5-formyl-2,4-dimethyl-1H-pyrrole-3-carboxamide
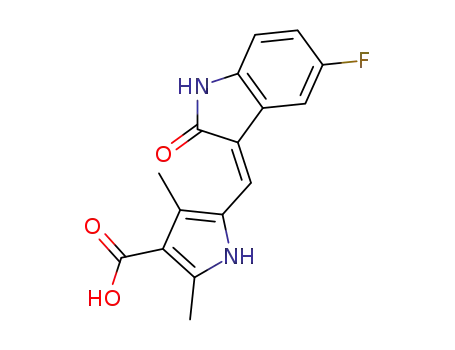
5-((Z)-(5-fluoro-2-oxoindolin-3-ylidene)methyl)-2,4-dimethyl-1H-pyrrole-3-carboxylic acid
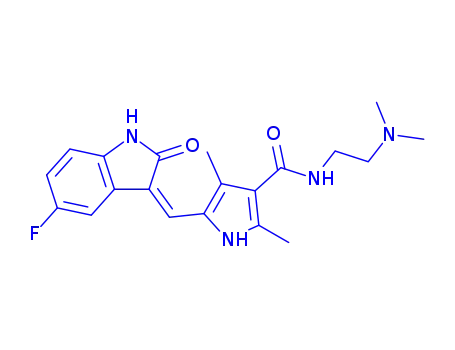
(Z)-N-(2-(dimethylamino)ethyl)-5-((5-fluoro-2-oxoindolin-3-ylidene)methyl)-2,4-dimethyl-1H-pyrrole-3-carboxamide
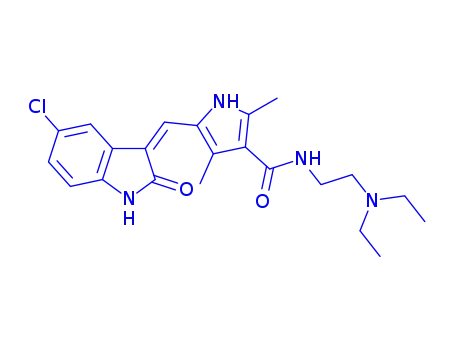
SU11652