- +86 185 8909 5435
- sales@senovaph.com
Your Location:Home >Products >Nucleoside and nucleotide food additives >114446-55-8

Purity:99%
|
Chemical Properties |
off-white to light yellow crystalline powder |
|
Uses |
Used in the synthesis of chiral boronic acids, which serve as reagents for enantiomeric purity determination of diols by H-NMR. |
InChI:InChI=1/C8H9BrO/c1-6(10)7-4-2-3-5-8(7)9/h2-6,10H,1H3/t6-/m0/s1
Preselection of 3,4-di-O-benzyl-D-manito...
Homogenous iridium complexes with asymme...
A highly efficient heterogeneous asymmet...
A series of Mn(I) catalysts containing i...
(Matrix presented) Chiral Ru-TsDPEN [N-(...
Chiral complex (1S,2S)-DPEN-RuCl2(TPP)2 ...
Carbonyl reductase BaSDR1 has been ident...
Alternaria alternata EBK-4 fungus isolat...
(R)-(+)-Limonene was transformed into mo...
The polymer-inorganic hybrid core-shell ...
The novel set of quinazoline-based chira...
Robust biocatalysts are in high demand f...
The asymmetric hydrogenation of aromatic...
The catalytic performance of ruthenium p...
cis-1,4-Butenediol is shown to be a high...
When complexed by selected ligands in ei...
The asymmetric transfer hydrogenation (A...
A nonenzymatic dynamic kinetic resolutio...
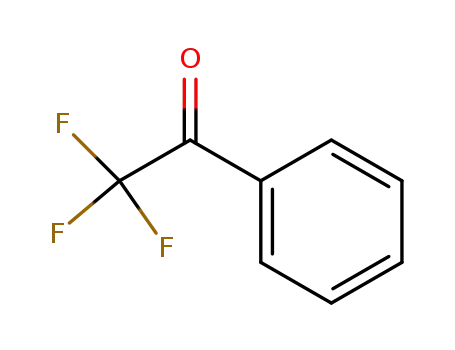
2,2,2-Trifluoroacetophenone

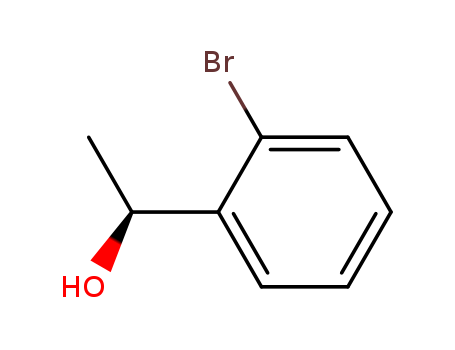
(1R)-1-(2-bromophenyl)ethan-1-ol

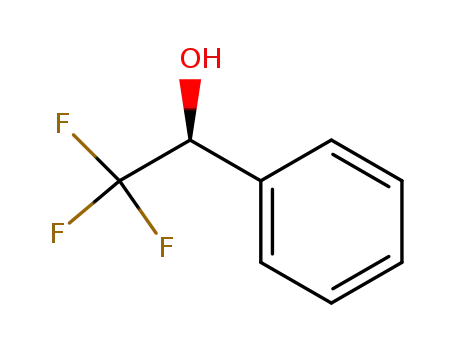
S(+)-1-phenyl-2,2,2-trifluoroethanol
| Conditions | Yield |
|---|---|
|
With ethanol; NADH; In aq. buffer; at 35 ℃; for 15h; pH=7; stereoselective reaction; Microbiological reaction; Enzymatic reaction;
|
32.3 % ee |
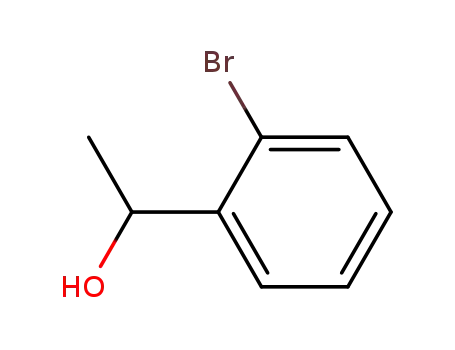
1-(2-bromophenyl)ethanol

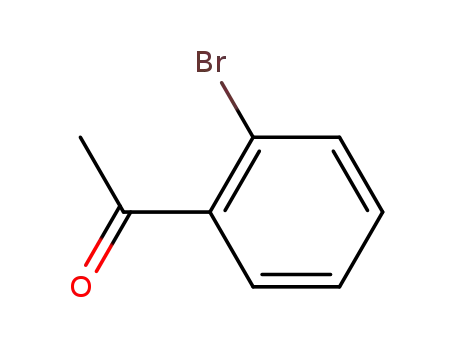
2-bromophenyl methyl ketone

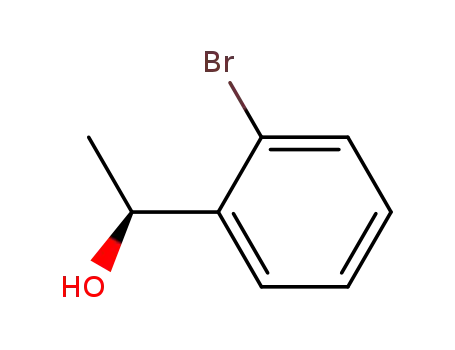
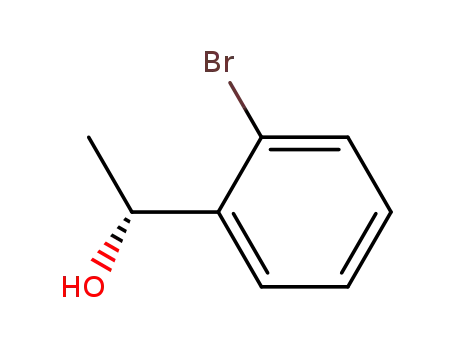
(1S)-1-(2-bromophenyl)ethanol


(1R)-1-(2-bromophenyl)ethan-1-ol
| Conditions | Yield |
|---|---|
|
With sodium hydrogencarbonate; sodium bromide; chiral 4,1'-dinaphthyl-3,2'-cyclo[C(Me)2-N(oxyl)-C(Me)2]; In dichloromethane; water; at -15 ℃; for 1.5h; Title compound not separated from byproducts; Electrolysis;
|
54% |
|
1-(2-bromophenyl)ethanol; With N,N'-bis(salicylidene)-1,2-cyclohexanediaminomanganese(III) chloride; potassium acetate; In dichloromethane; water; for 0.0833333h;
With N-Bromosuccinimide; In dichloromethane; water; at 20 ℃; for 4h; enantioselective reaction; Kinetics;
|
85 % ee |

2-bromophenyl methyl ketone

1-(2-bromophenyl)ethanol

dimethyl zinc(II)
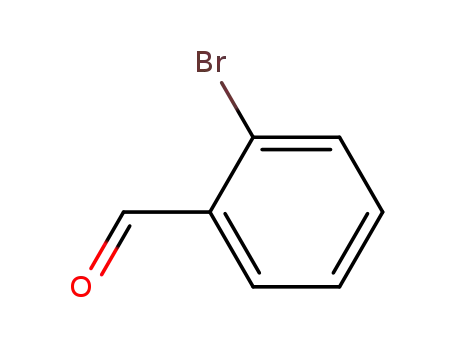
ortho-bromobenzaldehyde
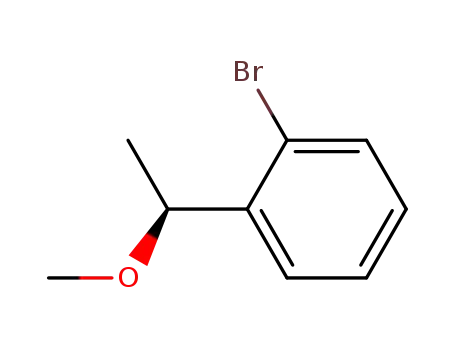
(S)-1-bromo-2-(1-methoxyethyl)benzene
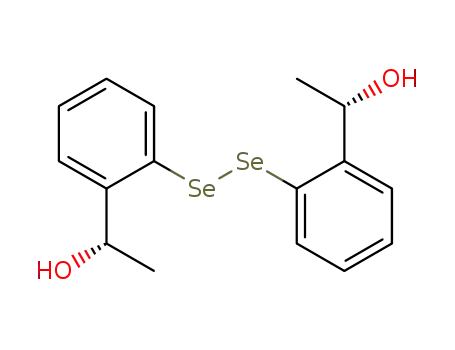
(S,S)-bis[2-(1-hydroxyethyl)phenyl] diselenide
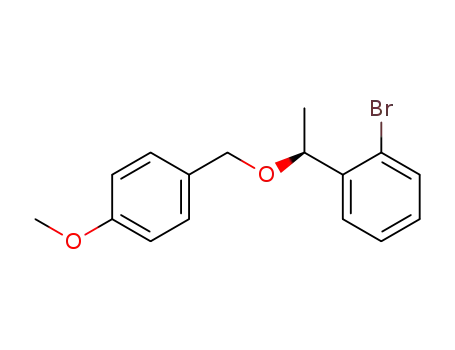
C16H17BrO2
(S)-[1-(2-bromophenyl)ethoxy]-tert-butyldimethylsilane