- +86 185 8909 5435
- sales@senovaph.com
Your Location:Home >Products >Organic Chemistry >162427-79-4
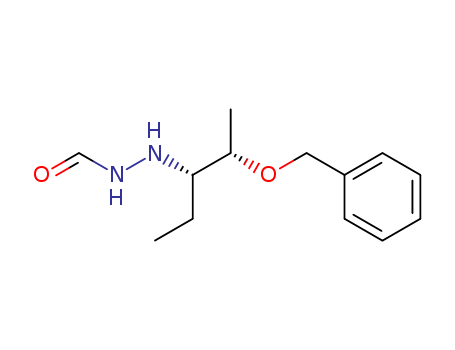

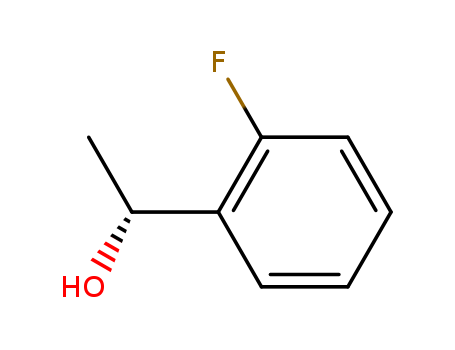
Purity:99%
|
Uses |
(R)-1-(2-Fluorophenyl)ethanol is a building block used in the synthesis of orally active lysophosphatidic acid receptor-1 antagonists. |
InChI:InChI=1/C8H9FO/c1-6(10)7-4-2-3-5-8(7)9/h2-6,10H,1H3/t6-/m1/s1
Four new chiral PNP′ pincer ligands with...
A short-mesochannel SBA-15 material func...
The invention discloses a novel non-meta...
A family of ferrocene-based chiral PNP l...
Bioreductions catalyzed by alcohol dehyd...
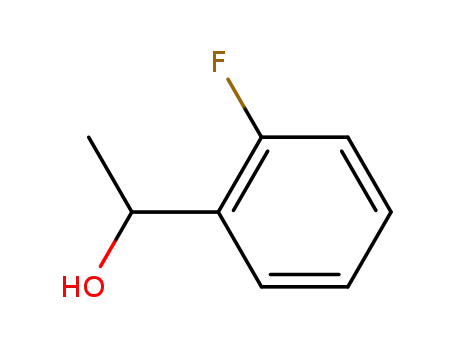
1-(2-fluorophenyl)ethanol

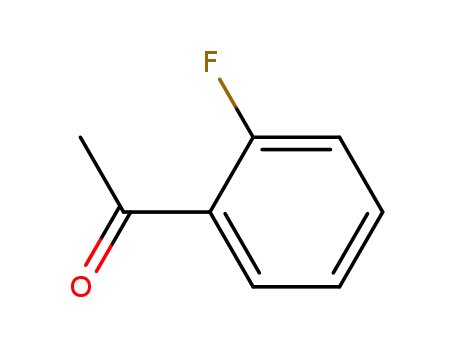
2'-Fluoroacetophenone

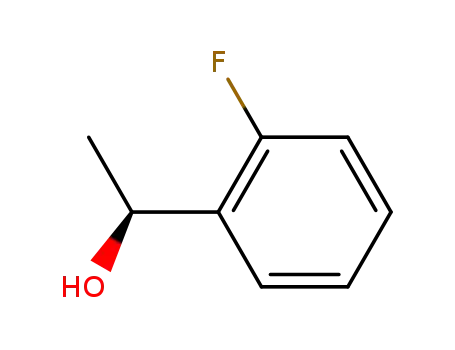
(1S)-1-(2-fluorophenyl)ethanol

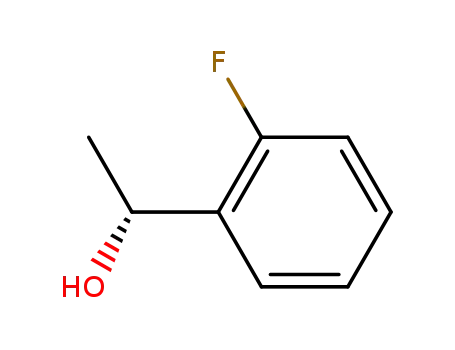
(R)-1-(2-fluorophenyl)ethanol
| Conditions | Yield |
|---|---|
|
With Arthrobacter atrocyaneus; In N,N-dimethyl-formamide; at 32 ℃; for 48h; Microbiological reaction;
|

1-(2-fluorophenyl)ethanol


2'-Fluoroacetophenone


(R)-1-(2-fluorophenyl)ethanol
| Conditions | Yield |
|---|---|
|
1-(2-fluorophenyl)ethanol; With C28H36ClMnN2O2; potassium acetate; In dichloromethane; water; for 0.0833333h;
With N-Bromosuccinimide; In dichloromethane; water; at 20 ℃; for 4h; enantioselective reaction; Kinetics;
|
94 % ee |

2'-Fluoroacetophenone
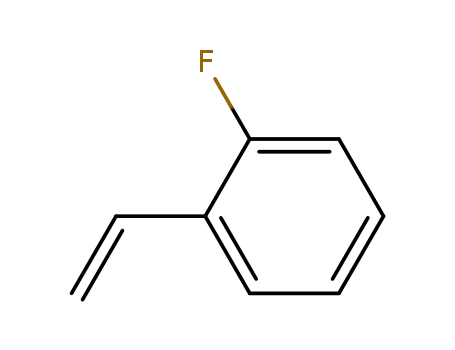
2-fluorostyrene

1-(2-fluorophenyl)ethanol
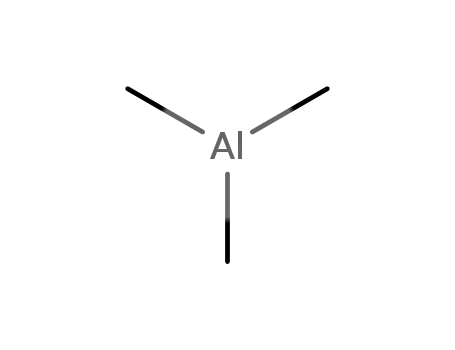
trimethylaluminum
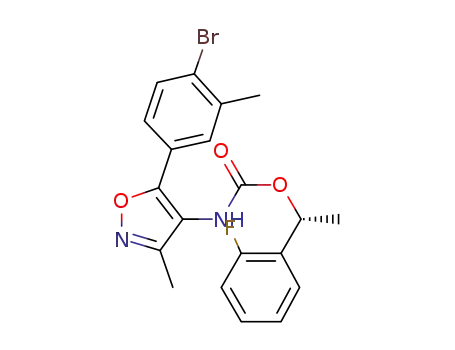
[5-(4-bromo-3-methyl-phenyl)-3-methyl-isoxazol-4-yl]-carbamic acid (R)-1-(2-fluoro-phenyl)-ethyl ester
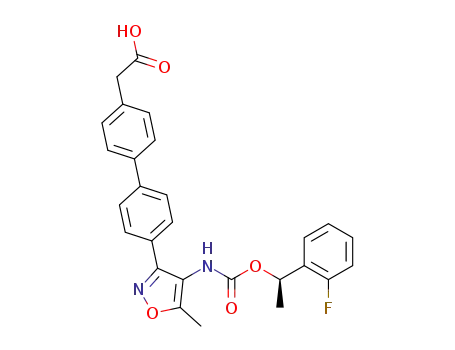
(4'-{4-[(R)-1-(2-fluoro-phenyl)-ethoxycarbonylamino]-5-methyl-isoxazol-3-yl}-biphenyl-4-yl)-acetic acid
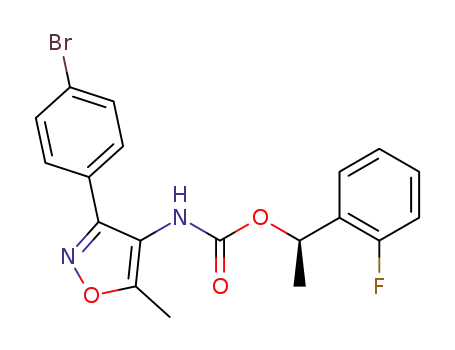
[3-(4-bromo-phenyl)-5-methyl-isoxazol-4-yl]-carbamic acid (R)-1-(2-fluoro-phenyl)-ethyl ester