SENOVA PHARMA
- +86 185 8909 5435
- sales@senovaph.com
Your Location:Home >Products >Active intermediate >697-64-3

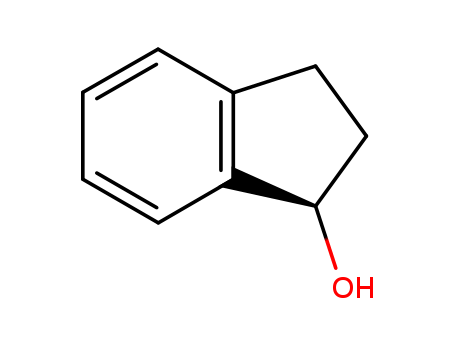
Purity:99%
|
Uses |
(R)-(-)-1-INDANOL is a building block used in pharmaceutical synthesis such as in the preparation of meropenem prodrugs for drug-resistant tuberculosis. |
InChI:InChI=1/C9H10O/c10-9-6-5-7-3-1-2-4-8(7)9/h1-4,9-10H,5-6H2/t9-/m1/s1
We report an OmpF loop deletion mutant, ...
The enantioselective oxidation of 2° alc...
A chiral cobalt pincer complex, when com...
1-Indanol

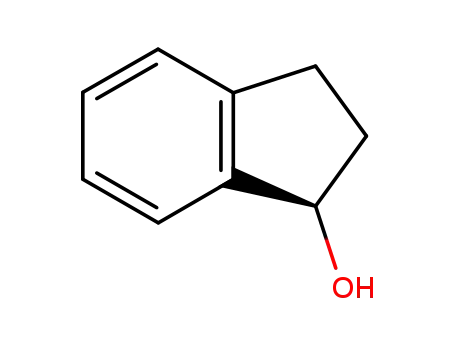
(R)-indan-1-ol

(S)-indanol

inden-1-one
| Conditions | Yield |
|---|---|
|
With palladium dichloro (η-2,5-norbornadiene); oxygen; (-)-sparteine; In toluene; at 60 ℃; for 54h; Title compound not separated from byproducts;
|
63% |
|
With 3 A molecular sieve; oxygen; (-)-sparteine; palladium dichloro (η-2,5-norbornadiene); In toluene; at 60 ℃; for 54h; under 760 Torr;
|
63% |
|
With (1R)-N-oxyl-1-(N-benzylcarbamoyl)-8-azabicyclo[3.2.1]octane; sodium hydrogencarbonate; sodium bromide; In dichloromethane; water; at 0 ℃; optical yield given as %ee; enantioselective reaction; Electrochemical reaction;
|
53% |
|
With oxygen; (-)-sparteine; dichloro(norbornadiene)palladium(II); In toluene; at 60 ℃; for 54h; Further Variations:; Reagents; Product distribution;
|
67 % Spectr. |
|
With (1R,2S,9S)-11-methyl-13-oxa-7,11-diazatricyclo[7.3.1.02,7]tridecane; C11H20Br2N2OPd; oxygen; caesium carbonate; In chloroform; at 20 ℃; for 18h; optical yield given as %ee; Molecular sieve;
|
|
|
With (S,S)-(+)-N,N’-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediaminomanganese(III) chloride; [bis(acetoxy)iodo]benzene; tetraethylammonium bromide; In dichloromethane; at 23 ℃; for 0.5h; optical yield given as %ee; enantioselective reaction; Inert atmosphere;
|
|
|
With α-naphthol; C136H102Fe2N4O6; In toluene; at 50 ℃; for 23h; optical yield given as %ee;
|
|
|
With sodium hypochlorite; C38H56ClMnN2O2; bromine; potassium acetate; In dichloromethane; water; at 20 ℃; for 0.666667h; enantioselective reaction; Kinetics; Mechanism;
|
75.4 % ee |
|
With (1R,2R)-1,2-di(naphthalen-1-yl)ethane-1,2-diamine; tert.-butylhydroperoxide; potassium carbonate; In decane; dichloromethane; for 20h; Molecular sieve;
|
67 % ee |
|
With manganese(II) triflate; 1-Adamantanecarboxylic acid; C32H38N4O2; dihydrogen peroxide; In water; acetonitrile; at 0 ℃; for 2h; Optical yield = 50 %ee;
|
|
|
With [(2S,2’S)-1,1’-bis((3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl)methyl)-2,2’-bipyrrolidineMnII(OTf)2]; dihydrogen peroxide; In acetonitrile; at -10 ℃; Optical yield = 24 %ee; enantioselective reaction; Resolution of racemate;
|
|
|
1-Indanol; With C32H40MnN2O6(1+)*Cl(1-); In dichloromethane; water; for 0.0833333h; Resolution of racemate;
With [bis(acetoxy)iodo]benzene; tetraethylammonium bromide; In dichloromethane; water; at 0 ℃; for 0.5h; Overall yield = 32 %; enantioselective reaction;
|
88 % ee |
|
With N-Bromosuccinimide; potassium acetate; C44H48ClMnN2O2; In dichloromethane; water; at 20 ℃; for 6h; Reagent/catalyst; Resolution of racemate;
|
14 % ee |

inden-1-one


(R)-indan-1-ol


(S)-indanol
| Conditions | Yield |
|---|---|
|
With dimethylsulfide borane complex; (S)-2-(anilinodiphenylmethyl)pyrrolidine; In tetrahydrofuran; at 20 ℃; for 2h; enantioselective reaction;
|
99% |
|
With dimethylsulfide borane complex; In tetrahydrofuran; at 20 ℃; for 2h; enantioselective reaction;
|
96% |
|
With sprouted Pisum sativa; In water; at 25 ℃; for 58h; optical yield given as %ee; enantioselective reaction;
|
42% |
|
With sodium (2S,3R)-(+)-4-dimethylamino-1,2-diphenyl-3-methyl-2-butanol; zinc; In tetrahydrofuran; methyl cyclohexane; for 17h; Product distribution; other olefins, enantioselectivity;
|
|
|
With sodium (2S,3R)-(+)-4-dimethylamino-1,2-diphenyl-3-methyl-2-butanol; zinc; In tetrahydrofuran; for 17h; Product distribution; Ambient temperature; asymmetric reduction; other arylalkylketones;
|
|
|
With lithium aluminium tetrahydride; (-)-N-methylephedrine; N-ethyl-N-phenylamine; In diethyl ether; at -78 ℃; Yield given. Yields of byproduct given. Title compound not separated from byproducts;
|
|
|
With 2,2'-iminobis[ethanol]; (-)-diisopinocamphenylborane chloride; Yield given. Multistep reaction. Yields of byproduct given. Title compound not separated from byproducts; 1) THF, -25 deg C, 15 h; 2) ethyl ether, 2 h;
|
|
|
With sodium (2S,3R)-(+)-4-dimethylamino-1,2-diphenyl-3-methyl-2-butanol; zinc; In tetrahydrofuran; methyl cyclohexane; for 17h; Yield given. Yields of byproduct given. Title compound not separated from byproducts;
|
|
|
With (2S,3R)-(+)-4-Dimethylamino-1,2-diphenyl-3-methyl-2-butanol; sodium hydride; zinc(II) chloride; Yield given. Multistep reaction. Yields of byproduct given. Title compound not separated from byproducts; 1) THF, 2 h, reflux, 2) methylcyclohexane, 17 h, r.t.;
|
|
|
With borane-THF; (R)-1-(1'-amino-1'-phenylmethyl)cyclopentanol; In tetrahydrofuran; at 30 ℃; for 2h; Title compound not separated from byproducts;
|
|
|
With lithium aluminium tetrahydride; bis <(1R)-1-hydroxyphenyl-ethan-2-yl> amine; In tetrahydrofuran; at -100 ℃; for 6h; Yield given. Yields of byproduct given. Title compound not separated from byproducts;
|
|
|
With n-butyllithium; chiral titanocene 1,1'-binaphth-2,2'-diolate; phenylsilane; tetrabutyl ammonium fluoride; Yield given. Multistep reaction. Yields of byproduct given. Title compound not separated from byproducts; 1.) benzene, hexane, 1.6 d, 2.) benzene, hexane, THF;
|
|
|
With dimethylsulfide borane complex; (1R)-amino-(2S)-indanol; In tetrahydrofuran; for 1h; Yield given. Yields of byproduct given. Title compound not separated from byproducts;
|
|
|
With potassium hydroxide;
|
|
|
With borane-THF; (-)-(1R,2S)-1-amino-2-hydroxy-1,2,3,4-tetrahydronaphthalene; In tetrahydrofuran; at 45 ℃; for 2h; Title compound not separated from byproducts;
|
|
|
With Trimethyl borate; dimethylsulfide borane complex; (1S,3R,4R)-2-azabicyclo<2.2.1>heptane-3-bis(phenyl)methanol; In tetrahydrofuran; Yield given. Yields of byproduct given. Title compound not separated from byproducts; Ambient temperature;
|
|
|
With LiAlH4 partially decomposed with (-)-N-methylephedrine and 2-ethylaminopyridine; In diethyl ether; at -78 ℃; for 3h; Yield given. Yields of byproduct given. Title compound not separated from byproducts;
|
|
|
With (3aS)-1-methyl-3, 3-diphenyl-tetrahydro-pyrrolo[1,2-c][1,3,2]oxazaborole borane complex; dimethylsulfide borane complex; In dichloromethane; at -20 ℃; Yield given. Yields of byproduct given;
|
|
|
With dimethylsulfide borane complex; chiral diphenyloxazaborolidine; In tetrahydrofuran; at 25 ℃; Yield given. Yields of byproduct given. Title compound not separated from byproducts;
|
|
|
With lithium aluminium tetrahydride; bis<(1R)-1-hydroxy-1-phenylpentan-2-yl>amine; In tetrahydrofuran; at -100 ℃; for 6h; Yield given. Yields of byproduct given. Title compound not separated from byproducts;
|
|
|
With dimethylsulfide borane complex; (1S,2R)-(+)-cis-1-amino-2-hydroxy-1,2,3,4-tetrahydronaphthalene; In tetrahydrofuran; for 2h; Yield given. Yields of byproduct given. Title compound not separated from byproducts;
|
|
|
With borane-THF; (1S,2R)-1-amino-2-indanol; In tetrahydrofuran; at 45 ℃; for 2h; Title compound not separated from byproducts;
|
|
|
With lithium aluminium tetrahydride; (-)-N-methylephedrine; N-ethyl-N-phenylamine; In diethyl ether; at -78 ℃; for 3h; Yield given. Yields of byproduct given. Title compound not separated from byproducts;
|
|
|
With sodium formate; isopropyl alcohol; [(η6-p-cymene)Ru(L-Pro)]3(BF4)3; In water; at 83 ℃; for 2h;
|
|
|
inden-1-one; With diphenylsilane; bis(1,5-cyclooctadiene)rhodium(I) tetrafluoroborate; (S,S)-2,2''-bis{(R)-1-(di-n-butylphosphino)ethyl}-1,1''-biferrocene; In tetrahydrofuran; at -40 ℃; for 3h;
With methanol; potassium carbonate; In tetrahydrofuran; at 20 ℃; for 4h; Title compound not separated from byproducts;
|
|
|
With borane-THF; (S)-2-[(4-trifluoromethyl)anilinomethyl]indoline; In tetrahydrofuran; at -15 ℃; for 2h; Title compound not separated from byproducts;
|
|
|
With potassium hydroxide; isopropyl alcohol; (4S)-4,5-dihydro-4-phenyl-2-(1,2,3,4-tetrahydroquinolin-8-yl)oxazole; [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; at -20 ℃; for 64h; Title compound not separated from byproducts;
|
|
|
With copper (II)-fluoride; phenylsilane; (S)-(1,1'-binaphthalene)-2,2'-diylbis(diphenylphosphine); In toluene; at 20 ℃; for 14h; Title compound not separated from byproducts;
|
|
|
inden-1-one; With (((1S,2S)-2-t-BuS-c-hexyloxy)PPh2)-(2,5-norbornadiene)RhOTf; 1-naphthylphenylsilane; In tetrahydrofuran; at -20 ℃; for 2h;
With methanol; In tetrahydrofuran; at 20 ℃; for 0.5h; Title compound not separated from byproducts;
|
|
|
With sodium aluminum tetrahydride; (S,S)-taddol; In tetrahydrofuran; at -20 ℃; Further Variations:; Reagents; Temperatures; Product distribution;
|
|
|
With formic acid; [RuCl2-(R,R)-NH3ClC(H)PhC(H)PhNHSO2CH2CH2Ph]2; triethylamine; In isopropyl alcohol; at 28 ℃; for 14h; Title compound not separated from byproducts;
|
|
|
With N-(p-toluenesulfonyl)-(1R,2R)-diphenylethylenediamine; sodium formate; [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; at 40 ℃; for 2h; Further Variations:; Reagents; Time; Product distribution;
|
|
|
With (R,R,R,R)-N,N,N',N'-(2-OH-2-(Ph)ethyl)4-1,3-xylylenediamine; SmI(η8-cyclooctatetraene)(thf); isopropyl alcohol; at 25 ℃; for 24h; Title compound not separated from byproducts;
|
|
|
With C49H47O2N2(1+)*BF4(1-); diphenylsilane; silver trifluoromethanesulfonate; tris(triphenylphosphine)ruthenium(II) chloride; In tetrahydrofuran; at 0 ℃; for 18h; Title compound not separated from byproducts;
|
|
|
With potassium tert-butylate; hydrogen; S-Xyl-P-PhosRuCl2*(S,S)-4,5-(NH2CH2)2-2,2-Me2-1,3-dioxolane; In isopropyl alcohol; at 25 - 30 ℃; under 7500.6 Torr; Title compound not separated from byproducts;
|
|
|
With [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; poly(ethylene glycol)-N-pTos-1,2-diphenylethylenediamine; sodium formate; In water; at 40 ℃; for 3h; Title compound not separated from byproducts;
|
|
|
L-threonine based helical poly(N-propargylamide)-Ru catalyst; In methanol; isopropyl alcohol; at 50 ℃; for 24h; Product distribution;
|
|
|
With dimethylsulfide borane complex; (S)-prolinol core-containing second-generation dendrimer; In tetrahydrofuran; for 0.8h; Title compound not separated from byproducts;
|
|
|
With potassium tert-butylate; hydrogen; [bis(2-methylallyl)cycloocta-1,5-diene]ruthenium(II); (R)-Ph-BINAN-H-Py; In isopropyl alcohol; at 25 ℃; for 18h; under 38000 Torr; Title compound not separated from byproducts;
|
|
|
With air; sodium formate; Ru-(1R,2R)-N-(p-toluenesulfonyl)-1,2-cyclohexanediamine; at 40 ℃; for 0.333333h; Title compound not separated from byproducts;
|
|
|
inden-1-one; With methylphenylsilane; sodium t-butanolate; (R)-BINAP-Cu(I); In toluene; at -78 ℃; for 18h;
With sodium hydroxide; In methanol; toluene; at 20 ℃; for 1h; Title compound not separated from byproducts;
|
|
|
inden-1-one; With copper (II)-fluoride; phenylsilane; (S)-(1,1'-binaphthalene)-2,2'-diylbis(diphenylphosphine); In toluene; at 20 ℃; for 3h;
With hydrogenchloride; Further stages. Title compound not separated from byproducts.;
|
|
|
With (1S,3R,4R)-2-azabicyclo[2.2.1]heptane-3-(R)-methylmethanol; [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; In isopropyl alcohol; at 20 ℃; for 18h; Title compound not separated from byproducts.;
|
|
|
[ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; (R,R)-1,2-bis(3',3"-PEG-oxyphenyl)-1-amino-2-(TsNH)-ethylene; In formic acid; triethylamine; at 50 ℃; for 25h;
|
|
|
With dimethylsulfide borane complex; In tetrahydrofuran; at 20 ℃; for 24h; Title compound not separated from byproducts.;
|
|
|
With hydrogen; chloro([(S,2S)-(?)-2-amino-1,2-diphenylethyl](4-toluenesulfonyl)amido)(mesitylene)ruthenium (II); In ethanol; at 30 ℃; for 11h; under 38002.6 Torr; Product distribution / selectivity;
|
|
|
With sodium formate; [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; In water; at 20 ℃; for 2h; Title compound not separated from byproducts.;
|
|
|
With sodium formate; [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; In water; at 20 ℃; Title compound not separated from byproducts.;
|
|
|
With diethoxymethylane; Fe(tetraphenyl-carBPI)(OAc); In tetrahydrofuran; at 40 ℃; for 40h; optical yield given as %ee; enantioselective reaction; Inert atmosphere;
|
|
|
With dimethyl sulfide borane; (2S)-2-(anilinomethyl)pyrrolidine; In tetrahydrofuran; at 20 ℃; for 2h; optical yield given as %ee; enantioselective reaction; Inert atmosphere;
|
|
|
With (2S)-N-[(1R,2S)-2,3-dihydro-2-hydroxy-1H-inden-1-yl]pyrrolidine-2-carboxamide; dichloro(p-cymene)ruthenium(II) dimer; sodium formate; In water; at 30 ℃; for 20h; optical yield given as %ee; Inert atmosphere;
|
|
|
With dichloro(pentamethylcyclopentadienyl)rhodium (III) dimer; (S)-valinol; diethylene glycol dibutyl ether; potassium isopropoxide; In tetrahydrofuran; isopropyl alcohol; at 20 ℃; for 2h; optical yield given as %ee;
|
|
|
With ECOENG500; [RuCl(p-cymene)TsDPEN]; water; sodium formate; at 20 - 50 ℃; optical yield given as %ee;
|
|
|
inden-1-one; With diphenylsilane; diethylzinc; C42H60N6; In hexane; toluene; at 20 ℃; for 24h; Inert atmosphere;
With sodium hydroxide; In methanol; hexane; toluene; at 20 ℃; for 1h; optical yield given as %ee; enantioselective reaction; Inert atmosphere;
|
|
|
inden-1-one; With phenylsilane; copper diacetate; (S)-4-phenyl-4,5-dihydro-3H-dinaphtho<2,1-c;1',2'-e>phosphepine; In toluene; at -20 ℃; for 5h; Inert atmosphere;
With methanol; tetrabutyl ammonium fluoride; In tetrahydrofuran; for 2h; optical yield given as %ee; enantioselective reaction;
|
|
|
With [RhCl2(p-cymene)]2; [(1R,3S)-6,7-dimethoxy-1-phenyl-1,2,3,4-tetrahydroisoquinolin-3-yl]methanol; potassium tert-butylate; isopropyl alcohol; at 20 ℃; for 24h; optical yield given as %ee; Inert atmosphere;
|
|
|
With [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; tetrabutylammomium bromide; water; sodium formate; (2S)-pyrrolidine-2-carboxylic acid N-((1R)-1-phenyl-ethyl)-amide; at 80 ℃; for 36h; optical yield given as %ee; enantioselective reaction;
|
|
|
With chloro(1,5-cyclooctadiene)rhodium(I) dimer; C7H7O3S(1-)*C9H14N3O(1+); potassium tert-butylate; potassium hydroxide; In isopropyl alcohol; at 80 ℃; for 20h; Inert atmosphere;
|
|
|
Multi-step reaction with 2 steps
1.1: iron(II) acetate; 1,2-bis((2S,5S)-2,5-dimethylphospholano)benzene / tetrahydrofuran / 65 °C / Inert atmosphere
1.2: 45 h / 20 °C / Inert atmosphere
2.1: water; sodium hydroxide / tetrahydrofuran; methanol / 0 - 20 °C / Inert atmosphere
With water; iron(II) acetate; 1,2-bis((2S,5S)-2,5-dimethylphospholano)benzene; sodium hydroxide; In tetrahydrofuran; methanol;
|
|
|
With (2S)-N-{(1R,2R)-2-[(S)-pyrrolidine-2-carboxamido]-1,2-diphenylethyl}pyrrolidine-2-carboxamide; [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; sodium formate; In water; at 60 ℃; for 24h; optical yield given as %ee; enantioselective reaction; Inert atmosphere;
|
|
|
With [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; 2-((3R,3aS,6R,6aR)-3-(benzyloxy)hexahydrofuro[3,2-b]furan-6-ylamino)ethanol; potassium tert-butylate; isopropyl alcohol; at 25 ℃; for 3h; optical yield given as %ee; enantioselective reaction; Inert atmosphere;
|
|
|
inden-1-one; With diethylzinc; 2,6-bis[(4R)-5,5-dihydro-4-phenyl-2-oxazolyl]pyridine; In tetrahydrofuran; toluene; at 20 ℃; for 20h; Inert atmosphere;
With sodium hydroxide; In tetrahydrofuran; methanol; diethyl ether; toluene; for 0.5h; optical yield given as %ee; enantioselective reaction; Inert atmosphere;
|
|
|
With chlorobis(cyclooctene)-iridium(I) dimer; (S)-N-(2-(tert-butylsulfinyl)benzyl)-1-(pyridin-2-yl)methanamine; potassium tert-butylate; isopropyl alcohol; In dichloromethane; at 20 ℃; for 3h; optical yield given as %ee; stereoselective reaction;
|
|
|
With diethyl zinc; diphenylsilane; (2R,3R,12R,13R,22R,23R)-1,4,11,14,21,24-hexaaza-(2,3:12,13:22,23)-tributano-(6,9:16,19:26,29)-trietheno-(1H,2H,3H,4H,5H,10H,11H,12H,13H,14H,15H,20H,21H,22H,23H,24H,25H,30H)-octadecahydro-(30)-annulene; In hexane; toluene; at 20 ℃; for 24h; optical yield given as %ee; enantioselective reaction; Inert atmosphere;
|
|
|
With ((N,N)-bis(2-(tert-butylthio-kS)benzylidene)-1,2-cyclohexyldiamino-kN,kN')dichlororuthenium(II); potassium tert-butylate; hydrogen; In isopropyl alcohol; at 60 ℃; for 2h; under 37503.8 Torr; Overall yield = 99 %Chromat.; Optical yield = 80 %ee; Inert atmosphere; Autoclave;
|
|
|
With bis(1,5-cyclooctadiene)diiridium(I) dichloride; (R)-N-(3-methylpyridine-2-methyl)-7-bis-(3,5-di-tert-butylphenyl)phosphino-7′-amino-1,1′-spirodihydroindane; potassium tert-butylate; hydrogen; In ethanol; at 20 ℃; for 1.5h; under 6080.41 - 7600.51 Torr; Overall yield = 94 %; Inert atmosphere; Sealed tube;
|
|
|
inden-1-one; With lithium aluminium tetrahydride; C37H45BrFeNO2P2(3+)*BF4(1-); hydrogen; In tetrahydrofuran; at 50 ℃; for 0.0833333h; under 3800.26 Torr;
In tetrahydrofuran; tert-Amyl alcohol; for 0.166667h;
With potassium tert-butylate; In tetrahydrofuran; enantioselective reaction; Catalytic behavior; Inert atmosphere; Glovebox;
|
22 % ee |
|
With C44H52FeN4P2(2+)*2BF4(1-); sodium t-butanolate; In isopropyl alcohol; at 75 ℃; for 15h; Overall yield = 93 %; Overall yield = 68.7 %Chromat.; enantioselective reaction; Glovebox; Schlenk technique; Inert atmosphere;
|
44.2 % ee |
|
With medlar fruit Mespilus germanica L.; In water; at 30 ℃; for 48h; Overall yield = 38 %; enantioselective reaction; Green chemistry; Enzymatic reaction;
|
25 % ee |
|
With bis(1,5-cyclooctadiene)diiridium(I) dichloride; (R)-N-(3-methylpyridine-2-methyl)-7-bis-(3,5-di-tert-butylphenyl)phosphino-7′-amino-1,1′-spirodihydroindane; potassium tert-butylate; hydrogen; In ethanol; at 20 ℃; for 1.5h; under 6080.41 - 7600.51 Torr; Overall yield = 94%; Optical yield = 72%ee; Autoclave;
|
|
|
With (2S)-N-[(1R,2S)-2,3-dihydro-2-hydroxy-1H-inden-1-yl]pyrrolidine-2-carboxamide; (p-cymene)ruthenium(II) chloride; sodium formate; In water; at 30 ℃; for 20h; Reagent/catalyst; Overall yield = 85 %; enantioselective reaction;
|
88 % ee |
|
With (p-cymene)ruthenium(II) chloride; (1R,2S)-1-Amino-2-indanol; sodium formate; In water; at 30 ℃; for 4h; Overall yield = 87 %; enantioselective reaction;
|
81 % ee |
|
With trimethylamine-N-oxide; C38H38FeO6Si2; hydrogen; In water; isopropyl alcohol; at 70 ℃; for 18h; under 22502.3 Torr; enantioselective reaction; Inert atmosphere; Autoclave; Schlenk technique;
|
59 % ee |
|
inden-1-one; With [dibenzhydryl-(S)-tBu-(iminopyridine-oxazoline)]FeBr2; diphenylsilane; sodium triethylborohydride; In toluene; at 25 ℃; for 3h; Inert atmosphere; Schlenk technique;
With sodium hydroxide; In methanol; water; toluene; for 10h; Overall yield = 64 %; Overall yield = 43 mg; enantioselective reaction;
|
76 % ee |
|
With dichloro(pentamethylcyclopentadienyl)rhodium (III) dimer; C16H20N2O2S; sodium formate; In water; at 25 ℃; for 1.5h; Optical yield = 84 %ee; enantioselective reaction; Catalytic behavior;
|
|
|
With trimethylamine-N-oxide; C38H38FeO6Si2; hydrogen; In water; isopropyl alcohol; at 70 ℃; for 18h; under 22502.3 Torr; enantioselective reaction; Autoclave;
|
59 % ee |
|
With diborane; (3aR)-1-methyl-3,3-diphenyl-tetrahydro-pyrrolo[1,2-c][1,3,2]oxazaborole; In tetrahydrofuran; 2-methyltetrahydrofuran; at 0 ℃; Overall yield = 40 %; Overall yield = 32 mg; enantioselective reaction; Flow reactor; Green chemistry;
|
68 % ee |
|
With diphenylsilane; C48H72N6O6; diethylzinc; In toluene; at 20 ℃; for 24h; Optical yield = 87 %ee; enantioselective reaction;
|
|
|
With C21H30ClN2Ru(1+)*Cl(1-); sodium formate; In water; at 60 ℃; for 12h; Reagent/catalyst; Time; Overall yield = 19 %; enantioselective reaction; Inert atmosphere; Schlenk technique;
|
48 % ee |
|
With tris(triphenylphosphine)ruthenium(II) chloride; (3aR,5R,7R,7aS)-2-(2-(diphenylphosphino)phenyl)-6,6-dimethyl-3a,4,5,6,7,7a-hexahydro-5,7-methanobenzo[d]oxazole; potassium tert-butylate; isopropyl alcohol; for 0.5h; Reagent/catalyst; Overall yield = 96 %; enantioselective reaction; Inert atmosphere; Reflux;
|
87 % ee |
|
With lithium aluminium tetrahydride; tert-Amyl alcohol; trans-(S,S)-[Fe(Ph2PCH(Ph)CH(Me)NCHCH2PCy2)(C0)2(Br)][BF4]; potassium tert-butylate; hydrogen; In tetrahydrofuran; at 50 ℃; for 18h; under 3800.26 Torr;
|
80 % ee |
|
With C19H33MnNO3P2(1+)*Br(1-); potassium tert-butylate; hydrogen; In 1,4-dioxane; at 30 ℃; for 4h; under 22502.3 Torr; Optical yield = 84 %ee; enantioselective reaction; Autoclave;
|
|
|
With D-glucose; NADH; In dimethyl sulfoxide; at 37 ℃; pH=6; enantioselective reaction; Enzymatic reaction;
|
60.7 % ee |
|
With C17H38BFeNOP2; hydrogen; In ethanol; at 30 ℃; for 3h; under 22502.3 Torr; Reagent/catalyst; Solvent; Temperature; Optical yield = 71 %ee; Autoclave;
|
|
|
With [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; sodium formate; chitosan; In water; isopropyl alcohol; at 20 ℃; for 70h; enantioselective reaction; Green chemistry;
|
88 % ee |
|
With C17H27Cl2NRuS2; potassium hydroxide; In isopropyl alcohol; for 12h; Reagent/catalyst; Overall yield = 84 %; Inert atmosphere; Schlenk technique; Reflux;
|
7 % ee |
|
With C17H38BFeNOP2; hydrogen; In ethanol; at 30 ℃; for 6h; under 22502.3 Torr; Overall yield = 81 %; enantioselective reaction; Autoclave;
|
63 % ee |
|
inden-1-one; With (R)-(+)-3,3'-bis(4-fluorophenyl)-[1,1'-binaphthalene]-2,2'-diol; dibutylmagnesium; 4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane; lithium chloride; In n-heptane; toluene; at -40 ℃; for 3h; Inert atmosphere; Sealed tube;
With methanol; In n-heptane; toluene; at -40 - 20 ℃; for 0.5h; Overall yield = 82 percent; Overall yield = 55 mg; enantioselective reaction; Inert atmosphere; Sealed tube;
|
82 % ee |
|
With 2,3,4,5,6-pentahydroxy-hexanal; carbonyl reductase from Kluyveromyces thermotolerans; In aq. phosphate buffer; at 30 ℃; pH=6.5; Overall yield = 15 percentChromat.; enantioselective reaction; Enzymatic reaction;
|
73 % ee |
|
With bis(1,5-cyclooctadiene)diiridium(I) dichloride; formic acid; (αR,2S)-(-)-1-(2-diphenylphosphinobenzyl)-α-(2,2-dimethylpropynyl)-2-pyrrolidinemethanol; potassium carbonate; at 40 ℃; for 6h; Overall yield = > 99 percent; Overall yield = 26.8 mg; enantioselective reaction; Inert atmosphere; Sealed tube;
|
87 % ee |
|
inden-1-one; With C51H80N4O4; scandium tris(trifluoromethanesulfonate); In tetrahydrofuran; at 35 ℃; for 0.5h;
With potassium borohydride; In tetrahydrofuran; water; at 0 ℃; for 8h; Overall yield = 99 %; enantioselective reaction;
|
86 % ee |
|
With hydrogen triethylboron; hydrogen; (R)-CoCl2[4'-phenyl-5-OMeCH3PyCH2NH(CH2)2PPh2]; tris(5-ethyl-2-furanyl)phosphine; caesium carbonate; In diethyl ether; at 25 ℃; for 20h; under 30003 Torr; Overall yield = 96 percent; enantioselective reaction; Autoclave;
|
62 % ee |
|
With dichloro(benzene)ruthenium(II) dimer; potassium tert-butylate; hydrogen; (R)-N,N'-((1R,2R)-cyclohexane-1,2-diyl)bis(2-((R)-tert-butyl(methyl)phosphino)benzamide); In isopropyl alcohol; at 20 ℃; for 8h; under 22502.3 Torr; Overall yield = 92 percent; enantioselective reaction; Schlenk technique;
|
8 % ee |
|
With hydrogenchloride; dipotassium hydrogenphosphate; magnesium sulfate heptahydrate; D-glucose; ammonium citrate tribasic; In water; at 25 ℃; for 64h; Overall yield = 95 percent; enantioselective reaction; Microbiological reaction;
|
52 % ee |
|
With dipotassium hydrogenphosphate; magnesium sulfate heptahydrate; manganese(II) sulfate tetrahydrate; D-glucose; ammonium citrate tribasic; In ethanol; water; at 20 ℃; for 24h; pH=6; Reagent/catalyst; pH-value; Temperature; Time; Concentration; enantioselective reaction; Microbiological reaction;
|
87 % ee |
|
With tetraethylammonium bromide; C49H66N6O3S3; acetic acid; In water; acetonitrile; at 25 ℃; pH=5; Overall yield = 44.4 percent; enantioselective reaction; Electrochemical reaction;
|
29.8 % ee |
Isopropenyl acetate

1-Indanol
INDANE
2,3-dihydro-1H-inden-1-yl-acetate

1-Indanol
(S)-2,3-dihydro-1H-indene-1-carboxylic acid
methyl (S)-4-[(2,3-dihydro-1H-inden-1-yl)oxy]benzenepropanoate

inden-1-one