- +86 185 8909 5435
- sales@senovaph.com
Your Location:Home >Products >Organic Chemistry >100306-34-1

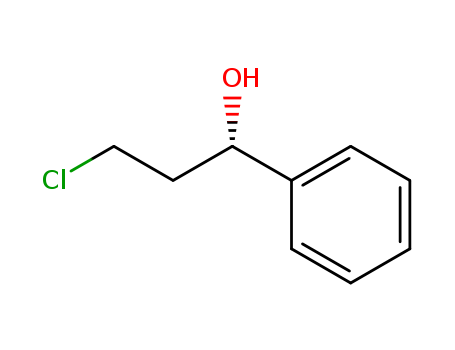
Purity:99%
|
Chemical Properties |
white to light yellow crystal powde |
|
Uses |
(S)-(-)-3-Chloro-1-phenyl-1-propanol (cas# 100306-34-1) is a compound useful in organic synthesis. |
InChI:InChI=1/C9H11ClO/c10-7-6-9(11)8-4-2-1-3-5-8/h1-5,9,11H,6-7H2/t9-/m0/s1
Microflow technology is established as a...
A broad range of prochiral ketones were ...
By enzyme screening, a ketoreductase clo...
Enantioselective reduction of ketones wi...
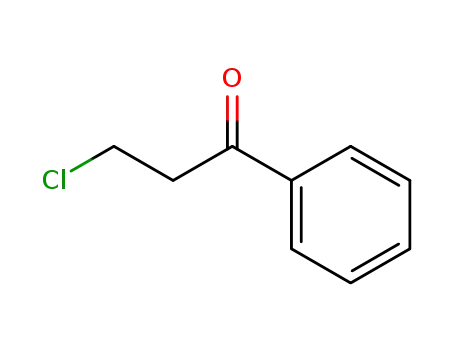
3-chloropropiophenone

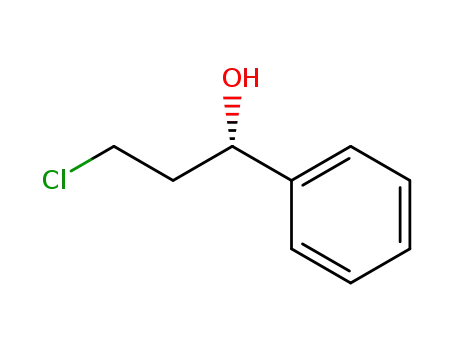
3-chloro-1-phenylpropanol

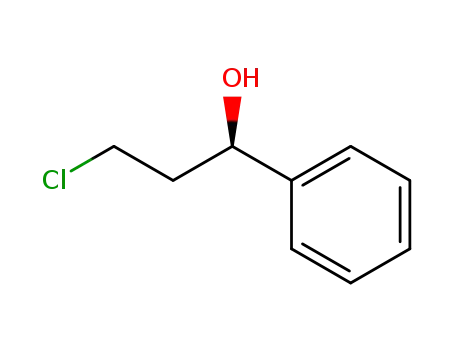
(1R)-3-chloro-1-phenylpropanol
| Conditions | Yield |
|---|---|
|
With dimethylsulfide borane complex; C23H22BNO3; In tetrahydrofuran; at 20 ℃; for 2h; Reagent/catalyst;
|
75% |
|
With dimethylsulfide borane complex; (+)-3-exo-amino-7,7-dimethoxynorbornan-2-exo-ol; In tetrahydrofuran; at 25 ℃; for 2h;
|
65% |
|
With dimethylsulfide borane complex; chiral diphenyloxazaborolidine; In tetrahydrofuran; at 25 ℃; Yield given. Yields of byproduct given. Title compound not separated from byproducts;
|
|
|
With lithium borohydride; N,N′-dibenzoyl-L-cysteine; tert-butyl alcohol; In tetrahydrofuran; at -78 - -30 ℃; for 9h; Yield given. Yields of byproduct given. Title compound not separated from byproducts;
|
|
|
With 9-borabicyclo[3.3.1]nonane dimer; borane-THF; (S)-diphenylprolinol; In tetrahydrofuran; at 20 ℃; for 1h; Title compound not separated from byproducts;
|
|
|
With borane; (2R,3S,4S,5R)-2,5-diamino-1,6-diphenyl-3,4-hexanediol; In tetrahydrofuran; at 35 ℃; for 5h; Title compound not separated from byproducts;
|
|
|
chiral benzodioxole-based copper; In tetrahydrofuran; toluene; tert-butyl alcohol; at -78 ℃; for 8h; Title compound not separated from byproducts;
|
|
|
With dimethylsulfide borane complex; In tetrahydrofuran; at 20 ℃; Title compound not separated from byproducts.;
|
|
|
With hydrogen; Cp*Ir(OTf)[(S,S)-Msdpen]; In methanol; at 60 ℃; for 24h; under 7600.51 Torr; Product distribution / selectivity;
|
77 % ee |
|
With ketoreductase 108; NADPH; at 30 ℃; pH=6.0; optical yield given as %ee; aq. phosphate buffer; Enzymatic reaction;
|
|
|
With borane N,N-diethylaniline complex; (S)-Corey-Bakshi-Shibata oxazaborolidine; In 1,2-dimethoxyethane; dichloromethane; water; at 25 - 30 ℃; for 12h; optical yield given as %ee; enantioselective reaction; Inert atmosphere;
|
|
|
With sodium t-butanolate; tert-butyl alcohol; (S)-2,2',6,6'-tetramethoxy-4,4'-bis(di(3,5-xylyl)phosphino)-3,3'-bipyridine; In toluene; at 0 ℃; for 14h; Overall yield = 90 %;
|
88 % ee |
|
With [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; (S,S,S,S)-N,N-bis(1,2-diphenylethylenediamino)-1,3-benzenedisulfonylamine; sodium formate; In water; at 60 ℃; for 11h; enantioselective reaction; Inert atmosphere;
|
85.2 % ee |
|
With diborane; (S)-1-methyl-3,3-diphenyl-hexahydropyrrolo[1,2-c][1,3,2]oxazaborole;
|
82 % ee |
|
With dimethylsulfide borane complex; (1R,2S,3R,5R)-2-(1',3',2'-dioxaborolan-2'-yloxy)apopinan-3-amine; In tetrahydrofuran; at 20 ℃; for 1h; Overall yield = 96 %; enantioselective reaction;
|
80 % ee |
|
With diborane; (3aR)-1-methyl-3,3-diphenyl-tetrahydro-pyrrolo[1,2-c][1,3,2]oxazaborole; In tetrahydrofuran; 2-methyltetrahydrofuran; at 0 ℃; Solvent; Temperature; Overall yield = 94 %; Overall yield = 92 mg; enantioselective reaction; Flow reactor; Green chemistry;
|
80 % ee |
|
With silver tetrafluoroborate; diethoxymethylane; C26H29N3O2*Cl(1-)*Ir(1+)*C8H12; at 20 ℃; for 20h; Overall yield = 82 %; stereoselective reaction;
|
66 % ee |
|
With D-glucose; dehydrogenase from Bacillus megaterium; ketoreductase cloned from Scheffersomyces stipitis CBS 6045; NADP; In aq. phosphate buffer; dimethyl sulfoxide; at 30 ℃; for 6h; pH=6.5; enantioselective reaction;
|
87.7 % ee |
|
With sodium tetrahydroborate; borane-THF; (R)-1,1'-Bi-2-naphthol; In tetrahydrofuran; at -78 - 24 ℃; for 12h; Overall yield = 49 percentSpectr.;
|

3-chloropropiophenone


3-chloro-1-phenylpropanol
| Conditions | Yield |
|---|---|
|
With potassium formate; Cp*IrCl[(S,S)-MsDPEN]; In water; toluene; at 50 ℃; for 24h; Product distribution / selectivity;
|
94% |
|
3-chloropropiophenone; With (S)-2,2',6,6'-tetramethoxy-4,4'-bis(diphenylphosphino)-3,3'-bipyridine; phenylsilane; copper(II) acetate monohydrate; In toluene; at -20 ℃; for 48h;
With hydrogenchloride; In water; toluene; Reagent/catalyst; enantioselective reaction;
|
94% |
|
With dimethylsulfide borane complex; (R)-2-[(1,3,2-dioxaborolan-2-yloxy)diphenylmethyl]pyrrolidine; In tetrahydrofuran; at 20 ℃; for 2h;
|
94% |
|
With dimethylsulfide borane complex; (R)-2-[(1,3,2-dioxaborolan-2-yloxy)diphenylmethyl]pyrrolidine; In tetrahydrofuran; at 20 ℃; for 2.16667h;
|
94% |
|
With borane N,N-diethylaniline complex; (3aR)-1-methyl-3,3-diphenyl-tetrahydro-pyrrolo[1,2-c][1,3,2]oxazaborole; In dichloromethane; toluene; at -7 - 20 ℃; for 0.166667h; optical yield given as %ee; enantioselective reaction;
|
88% |
|
With potassium phosphate; Candida tenuis xylose reductase; NADH; In methanol; for 24h;
|
80% |
|
3-chloropropiophenone; With borane N,N-diethylaniline complex; (3aR)-1-methyl-3,3-diphenyl-tetrahydro-pyrrolo[1,2-c][1,3,2]oxazaborole; In toluene; at 20 ℃; for 6h; Inert atmosphere;
With hydrogenchloride; In methanol; water; toluene; for 0.166667h; optical yield given as %ee; enantioselective reaction; Inert atmosphere;
|
79% |
|
With borane; (3aR)-1-methyl-3,3-diphenyl-tetrahydro-pyrrolo[1,2-c][1,3,2]oxazaborole; In tetrahydrofuran; optical yield given as %ee;
|
77% |
|
With (S)-2,2',6,6'-tetramethoxy-4,4'-bis(diphenylphosphino)-3,3'-bipyridine; phenylsilane; copper(II) acetate monohydrate; In toluene; at -20 ℃; for 24h; Inert atmosphere; Schlenk technique;
|
73% |
|
With potassium phosphate buffer; Rhodotorula sp. AS22241; at 30 ℃; for 20h; pH=7.0;
|
50% |
|
With dIpc2BCl; In tetrahydrofuran; at -25 ℃; Product distribution; other ring and chain substituted haloaralkyl ketones;
|
|
|
Multi-step reaction with 3 steps
1: 88 percent / NaBH4 / ethanol / 2 h / 20 °C
2: 93 percent / pyridine; DMAP / CH2Cl2 / 0 - 20 °C
3: aq. phosphate buffer; Novozyme 435 / 288 h / 30 °C / pH 7
With pyridine; dmap; sodium tetrahydroborate; phosphate buffer; novozyme 435; In ethanol; dichloromethane; 1: Reduction / 2: Esterification / 3: Hydrolysis;
|
|
|
Multi-step reaction with 2 steps
1: 88 percent / NaBH4 / ethanol / 2 h / 20 °C
2: 33 percent / Novozyme 435 / hexane / 192 h / 30 °C
With sodium tetrahydroborate; novozyme 435; In ethanol; hexane; 1: Reduction / 2: Esterification;
|
|
|
Multi-step reaction with 4 steps
1: LiAlH4 / diethyl ether
2: pyridine / CH2Cl2
4: 100 percent / K2CO3, MeOH
With pyridine; methanol; lithium aluminium tetrahydride; potassium carbonate; In diethyl ether; dichloromethane;
|
|
|
With (S)-2,2',6,6'-tetramethoxy-4,4'-bis(diphenylphosphino)-3,3'-bipyridine; phenylsilane; copper diacetate; In toluene; at -20 ℃; for 36h; stereoselective reaction;
|
|
|
With NADPH; 1-butyl-3-methylimidazolium trifluoromethanesulfonimide; In Tween-40; for 8h; pH=7 - 7.5; optical yield given as %ee; enantioselective reaction; Tris-HCl buffer; Enzymatic reaction;
|
100 mmol |
|
With yeast culture of Candida viswanathii KCh 120; In acetone; at 25 ℃; for 24h; enantioselective reaction; Microbiological reaction;
|
84 mg |
|
With potassium phosphate; Candida tenuis xylose reductase; NADH; In methanol; for 24h;
|

3-chloropropiophenone
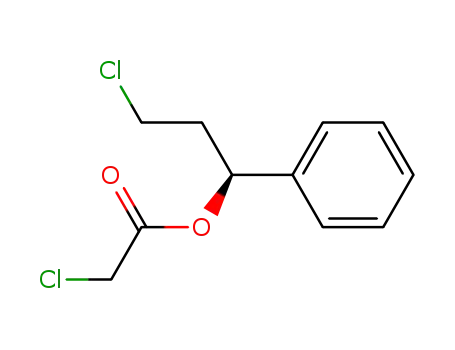
Chloro-acetic acid (S)-3-chloro-1-phenyl-propyl ester
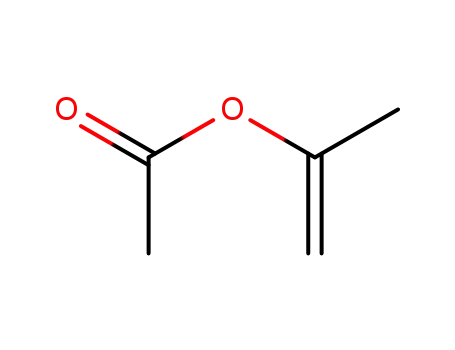
Isopropenyl acetate
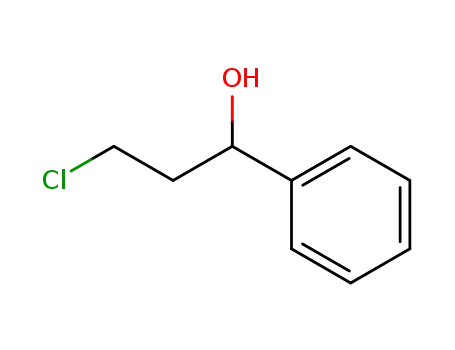
3-chloro-1-phenyl-propan-1-ol
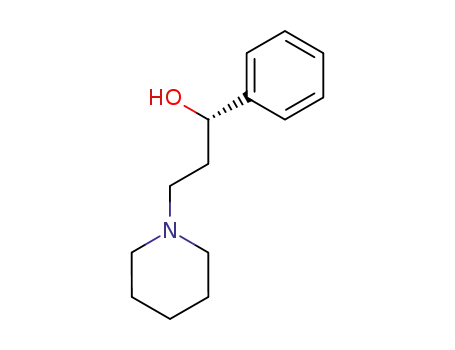
(S)-3-N-Piperidinyl-1-phenyl-1-propanol
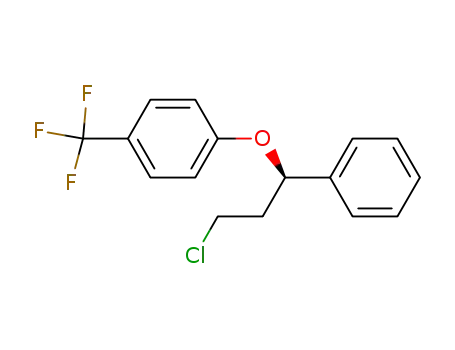
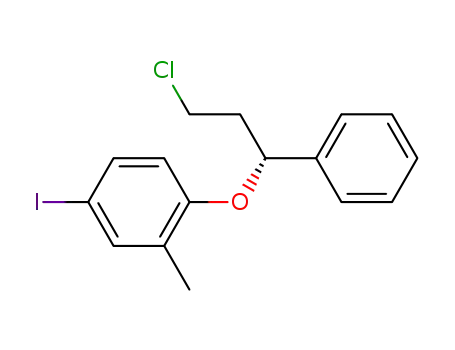
(R)-(+)-1-chloro-3-(4-iodo-2-methylphenoxy)-3-phenylpropane
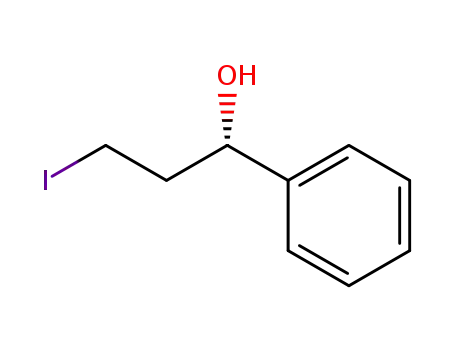
(S)-(-)-3-iodo-1-phenyl-1-propanol