- +86 185 8909 5435
- sales@senovaph.com
Your Location:Home >Products >Organic Chemistry >171032-87-4

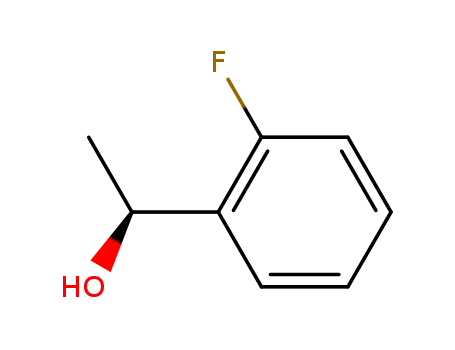
Purity:99%
|
Uses |
(S)-1-(2-FLUOROPHENYL)ETHANOL is a building block used in organic synthesis such as JN403, a novel nicotinic acetylcholine receptor α7 agonist. |
InChI:InChI=1/C8H9FO/c1-6(10)7-4-2-3-5-8(7)9/h2-6,10H,1H3/t6-/m0/s1
The immobilized resting-cell of Geotrich...
The invention discloses a novel non-meta...
The Noyori-Ikariya (arene)Ru(II)/TsDPEN ...
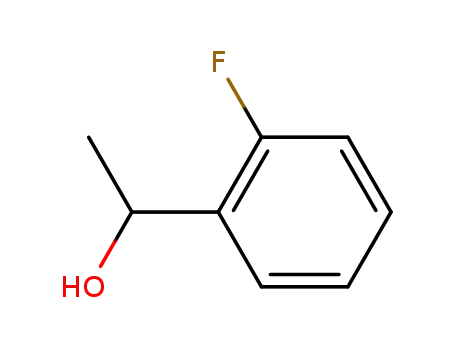
1-(2-fluorophenyl)ethanol

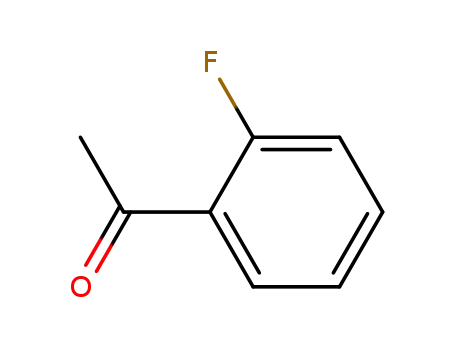
2'-Fluoroacetophenone

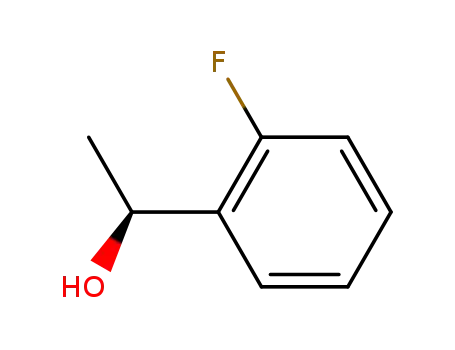
(1S)-1-(2-fluorophenyl)ethanol

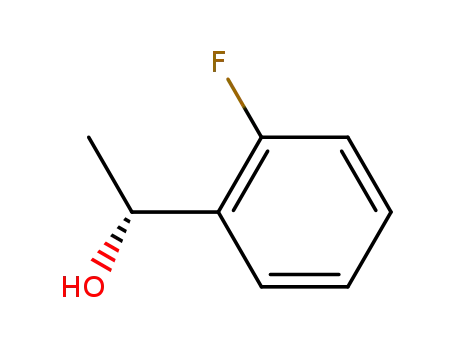
(R)-1-(2-fluorophenyl)ethanol
| Conditions | Yield |
|---|---|
|
With Arthrobacter atrocyaneus; In N,N-dimethyl-formamide; at 32 ℃; for 48h; Microbiological reaction;
|

2'-Fluoroacetophenone

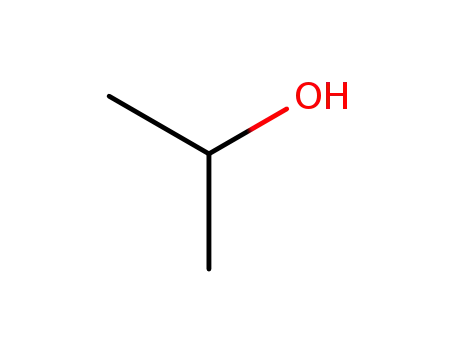
isopropyl alcohol

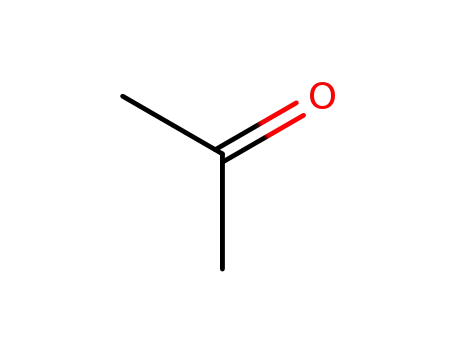
acetone


(1S)-1-(2-fluorophenyl)ethanol


(R)-1-(2-fluorophenyl)ethanol
| Conditions | Yield |
|---|---|
|
With C32H31Cl2N4OPRu; potassium isopropoxide; at 30 ℃; for 0.5h; under 750.075 Torr; optical yield given as %ee; enantioselective reaction; Inert atmosphere;
|
|
|
With potassium hydroxide; at 82 ℃; for 0.5h; Reagent/catalyst; enantioselective reaction; Catalytic behavior; Inert atmosphere; Schlenk technique;
|
45 % ee |

2'-Fluoroacetophenone
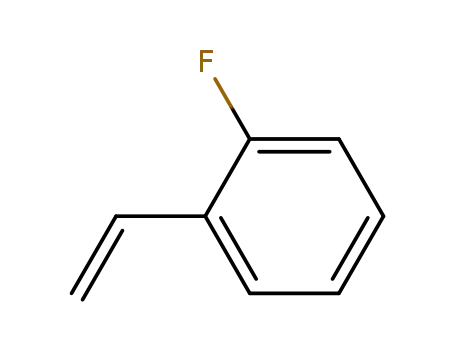
2-fluorostyrene

1-(2-fluorophenyl)ethanol
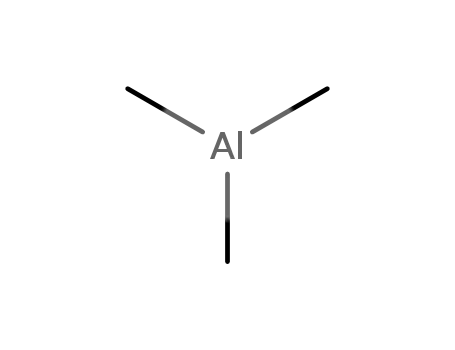
trimethylaluminum
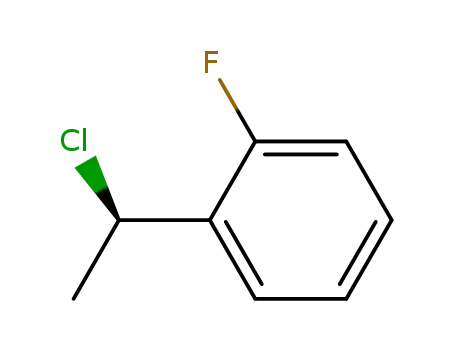
1-[(1R)-1-chloroethyl]-2-fluorobenzene
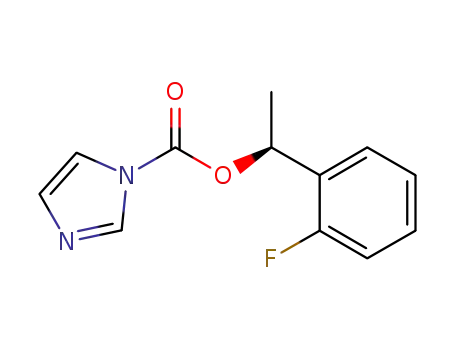
imidazole-1-carboxylic acid (S)-1-(2-fluorophenyl)ethyl ester
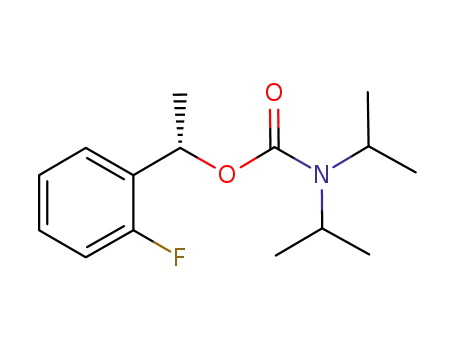
(S)-(-)-1-(2-fluorophenyl)ethyl N,N-diisopropylcarbamate
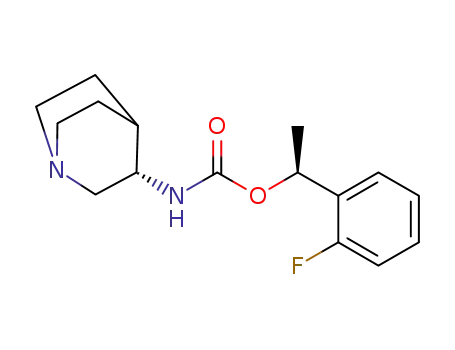
JN 403