SENOVA PHARMA
- +86 185 8909 5435
- sales@senovaph.com
Your Location:Home >Products >Pharmaceutical intermediates >42070-90-6

Purity:99%
A substoichiometric enantioselective ver...
The enantioselective hydroboration of vi...
Cyanobacteria Synechocystis sp. PCC 6803...
Most ligands applied for asymmetric hydr...
The invention discloses a tridentate nit...
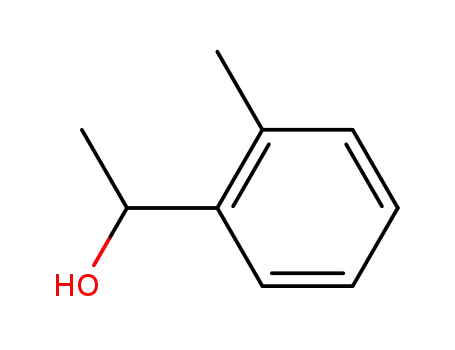
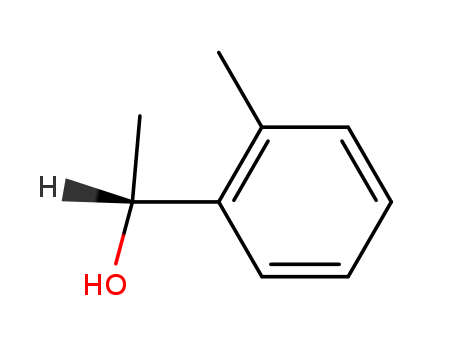
1-O-tolyl-ethanol

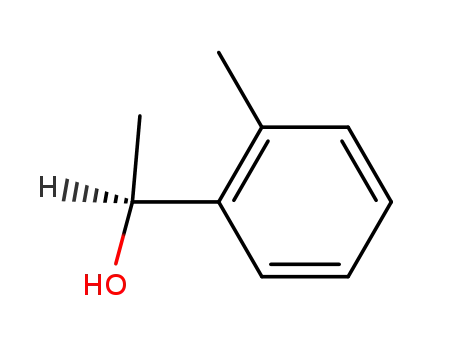
(S)-1-(2-Methylphenyl)ethanol

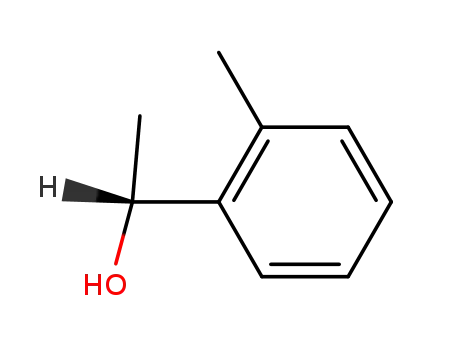
(R)-1-(2-methylphenyl)ethanol

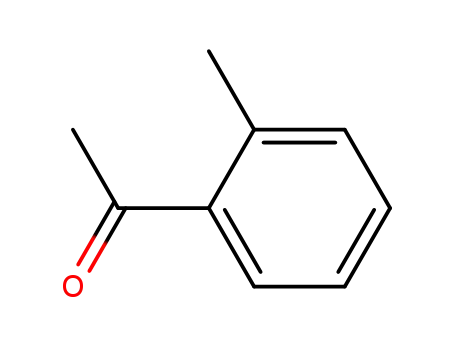
2-Methylacetophenone
| Conditions | Yield |
|---|---|
|
With (1R)-N-oxyl-1-(N-benzylcarbamoyl)-8-azabicyclo[3.2.1]octane; sodium hydrogencarbonate; sodium bromide; In dichloromethane; water; at 0 ℃; optical yield given as %ee; enantioselective reaction; Electrochemical reaction;
|
50% |
|
With palladium dichloro (η-2,5-norbornadiene); oxygen; (-)-sparteine; In toluene; at 80 ℃; for 144h; Title compound not separated from byproducts;
|
46% |
|
With 3 A molecular sieve; oxygen; (-)-sparteine; palladium dichloro (η-2,5-norbornadiene); In toluene; at 80 ℃; for 144h; under 760 Torr;
|
46% |
|
With sodium hypochlorite; potassium bromide; (-)-(S)-3,5-dihydro-3,3,5,5-tetramethyl-4H-dinaphth<2,1-c:1',2'-e>azepine-N-oxyl; In dichloromethane; water; at 0 ℃; for 0.5h; Title compound not separated from byproducts;
|
|
|
With [bis(acetoxy)iodo]benzene; (S,S)-7-aza-2,5-dimethylnorbornan-7-yloxy radical; In dichloromethane; at -35 ℃; for 3h; Further Variations:; Reagents; Product distribution;
|
|
|
1-O-tolyl-ethanol; With Br(1-)*C75H107Cl2Mn2N8O4(1+); potassium bromide; In dichloromethane; water; at 20 ℃; for 0.166667h;
With [bis(acetoxy)iodo]benzene; In dichloromethane; water; optical yield given as %ee;
|
|
|
1-O-tolyl-ethanol; With potassium bromide; In water; at 25 ℃; for 0.166667h; Resolution of racemate;
With [bis(acetoxy)iodo]benzene; In water; at 25 ℃; for 2h; enantioselective reaction;
|
|
|
1-O-tolyl-ethanol; With 36Zn(2+)*6O(2-)*12C40H44O12S4(4-)*12Mn(3+)*12C30H34N2O6(4-)*21H2O*38C3H7NO; In dichloromethane; water; for 0.0833333h; Resolution of racemate;
With [bis(acetoxy)iodo]benzene; tetraethylammonium bromide; In dichloromethane; water; at 0 ℃; for 0.5h; Reagent/catalyst; Optical yield = 36 %ee; enantioselective reaction;
|

1-O-tolyl-ethanol


(S)-1-(2-Methylphenyl)ethanol


(R)-1-(2-methylphenyl)ethanol


2-Methylacetophenone
| Conditions | Yield |
|---|---|
|
With (1R)-N-oxyl-1-(N-benzylcarbamoyl)-8-azabicyclo[3.2.1]octane; sodium hydrogencarbonate; sodium bromide; In dichloromethane; water; at 0 ℃; optical yield given as %ee; enantioselective reaction; Electrochemical reaction;
|
50% |
|
With palladium dichloro (η-2,5-norbornadiene); oxygen; (-)-sparteine; In toluene; at 80 ℃; for 144h; Title compound not separated from byproducts;
|
46% |
|
With 3 A molecular sieve; oxygen; (-)-sparteine; palladium dichloro (η-2,5-norbornadiene); In toluene; at 80 ℃; for 144h; under 760 Torr;
|
46% |
|
With sodium hypochlorite; potassium bromide; (-)-(S)-3,5-dihydro-3,3,5,5-tetramethyl-4H-dinaphth<2,1-c:1',2'-e>azepine-N-oxyl; In dichloromethane; water; at 0 ℃; for 0.5h; Title compound not separated from byproducts;
|
|
|
With [bis(acetoxy)iodo]benzene; (S,S)-7-aza-2,5-dimethylnorbornan-7-yloxy radical; In dichloromethane; at -35 ℃; for 3h; Further Variations:; Reagents; Product distribution;
|
|
|
1-O-tolyl-ethanol; With Br(1-)*C75H107Cl2Mn2N8O4(1+); potassium bromide; In dichloromethane; water; at 20 ℃; for 0.166667h;
With [bis(acetoxy)iodo]benzene; In dichloromethane; water; optical yield given as %ee;
|
|
|
1-O-tolyl-ethanol; With potassium bromide; In water; at 25 ℃; for 0.166667h; Resolution of racemate;
With [bis(acetoxy)iodo]benzene; In water; at 25 ℃; for 2h; enantioselective reaction;
|
|
|
1-O-tolyl-ethanol; With 36Zn(2+)*6O(2-)*12C40H44O12S4(4-)*12Mn(3+)*12C30H34N2O6(4-)*21H2O*38C3H7NO; In dichloromethane; water; for 0.0833333h; Resolution of racemate;
With [bis(acetoxy)iodo]benzene; tetraethylammonium bromide; In dichloromethane; water; at 0 ℃; for 0.5h; Reagent/catalyst; Optical yield = 36 %ee; enantioselective reaction;
|

tetramethyl titanium
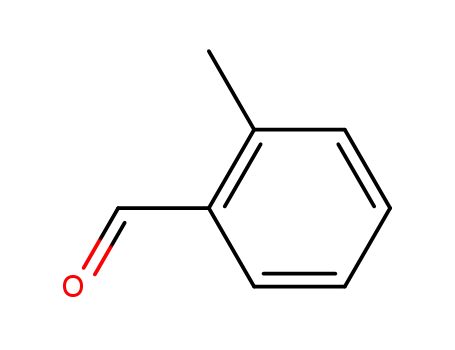
2-methylphenyl aldehyde

2-Methylacetophenone

1-O-tolyl-ethanol
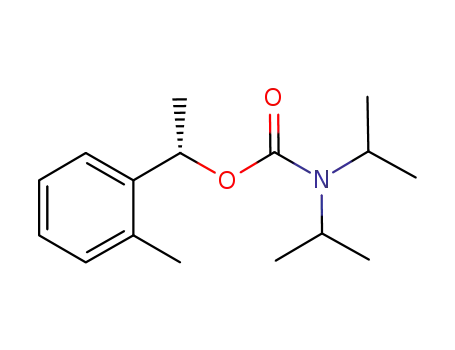
(S)-(-)-1-(2-methylphenyl)ethyl N,N-diisopropylcarbamate

1-O-tolyl-ethanol