- +86 185 8909 5435
- sales@senovaph.com
Your Location:Home >Products >Organic Chemistry >21950-36-7

Purity:99%
Four stable analogues of methionyl adeny...
Functional screening of structurally div...
Tyrosyl-tRNA synthetase (TyrRS) is an am...
A series of cycloalkyl substituted analo...
The invention discloses a purine derivat...
Viral mRNA cap methyltransferases (MTase...
Described herein, inter alia, are compou...
Rh/Al2O3 can be used as an effective che...
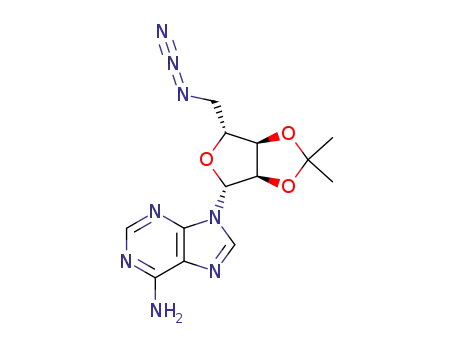
5'-azido-5'-deoxy-2',3'-O-isopropylidene adenosine

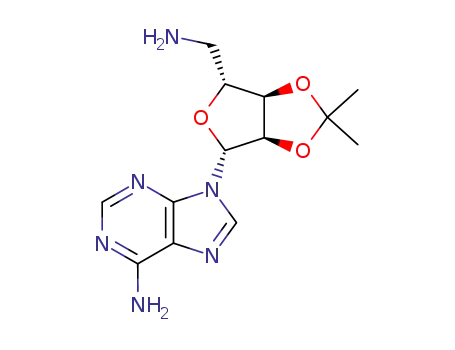
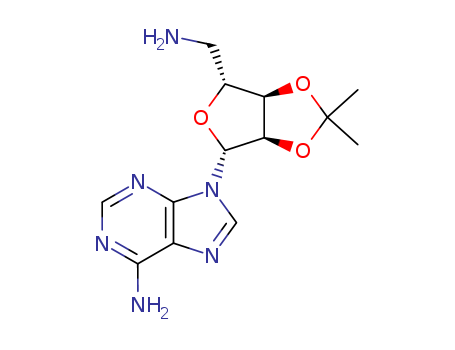
5'-amino-5'-deoxy-2',3'-O-isopropylideneadenosine
| Conditions | Yield |
|---|---|
|
With palladium 10% on activated carbon; hydrogen; In methanol;
|
100% |
|
With palladium on activated charcoal; hydrogen; In ethanol;
|
100% |
|
With hydrazine hydrate; In ethanol; at 20 ℃; chemoselective reaction;
|
98% |
|
With palladium 10% on activated carbon; hydrogen; In ethanol; at 20 ℃; for 16h;
|
95% |
|
With hydrogen; palladium on activated charcoal; In ethanol; at 20 ℃; for 5h;
|
90% |
|
With palladium on activated charcoal; hydrogen; In methanol; at 20 ℃; for 2.5h;
|
88% |
|
With triphenylphosphine; In pyridine; ammonium hydroxide; at 20 ℃;
|
84% |
|
With hydrogen; palladium on activated charcoal; In ethanol; for 5h; under 2327.2 Torr;
|
70% |
|
5'-azido-5'-deoxy-2',3'-O-isopropylidene adenosine; In tetrahydrofuran; at 20 ℃; for 4h; Inert atmosphere;
With water; In tetrahydrofuran; at 20 ℃; Inert atmosphere;
|
70% |
|
With palladium 10% on activated carbon; hydrogen; In ethanol; for 12h; under 760.051 Torr;
|
68% |
|
With Rh/Al2O3; hydrogen; acetic acid; In ethyl acetate; toluene; at 20 ℃; for 24h; under 760.051 Torr; chemoselective reaction;
|
65% |
|
With ammonium hydroxide; triphenylphosphine; In pyridine; for 18h; Yield given; Ambient temperature;
|
|
|
Multi-step reaction with 2 steps
1: tetrahydrofuran / 1 h / 20 °C
2: H2O / 60 °C
With water; In tetrahydrofuran; 1: Staudinger reaction;
|
|
|
With palladium 10% on activated carbon; hydrogen; In ethanol; at 20 ℃; for 18h;
|
2.21 g |
|
With palladium 10% on activated carbon; hydrogen; In methanol; at 20 ℃; for 4h;
|
|
|
With 5%-palladium/activated carbon; hydrogen;
|
|
|
With palladium 10% on activated carbon; hydrogen; In ethyl acetate; at 20 ℃; for 3h;
|
850 mg |
|
With palladium on activated charcoal; hydrogen; In methanol; for 4h;
|
![2-[[(3aR,4R,6R,6aR)-6-(6-amino-9H-purin-9-yl)-2,2-dimethyltetrahydro-2H-furo[3,4-d][1,3]dioxol-4-yl]methyl]-2,3-dihydro-1H-isoindol-1,3-dione](/upload/2023/5\2f25a38f-4dce-4888-a2a1-5fdb7e0ed205.png)
2-[[(3aR,4R,6R,6aR)-6-(6-amino-9H-purin-9-yl)-2,2-dimethyltetrahydro-2H-furo[3,4-d][1,3]dioxol-4-yl]methyl]-2,3-dihydro-1H-isoindol-1,3-dione


5'-amino-5'-deoxy-2',3'-O-isopropylideneadenosine
| Conditions | Yield |
|---|---|
|
With hydrazine hydrate; In ethanol; for 2h; Heating;
|
96% |
|
With ethanol; hydrazine hydrate; at 80 ℃; for 2h;
|
96.4% |
|
With hydrazine hydrate; In ethanol;
|
93% |
|
With hydrazine hydrate; In ethanol; Reflux;
|
90.5% |
|
With hydrazine hydrate; In ethanol; Reflux;
|
78% |
|
With hydrazine; In ethanol;
|
78% |
|
With methylhydrazine; In ethanol; at 15 - 40 ℃; for 18h;
|
78% |
|
With hydrazine hydrate; In ethanol; Heating;
|
77% |
|
With hydrazine hydrate; In ethanol; at 20 ℃; for 3h; Reflux;
|
76% |
|
With hydrazine hydrate; In ethanol; for 2h; Reflux;
|
64% |
|
With hydrazine hydrate; In ethanol; Reflux;
|
|
|
With hydrazine hydrate; In ethanol; at 80 ℃; for 1.5h;
|
|
|
With hydrazine hydrate; In ethanol; at 80 ℃; for 1.5h;
|
|
|
With hydrazine hydrate; In ethanol; Reflux;
|
|
|
With hydrazine hydrate; In ethanol; at 85 ℃; for 2h;
|
|
|
With hydrazine hydrate; In ethanol; at 80 ℃; for 2h;
|
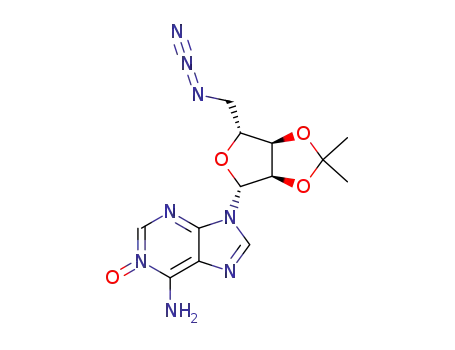
5'-azido-5'-deoxy-2',3'-O-isopropylideneadenosine N1-oxide
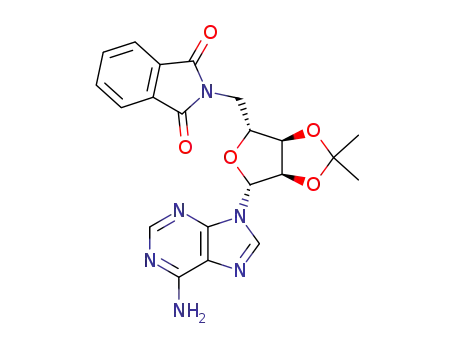
2-[[(3aR,4R,6R,6aR)-6-(6-amino-9H-purin-9-yl)-2,2-dimethyltetrahydro-2H-furo[3,4-d][1,3]dioxol-4-yl]methyl]-2,3-dihydro-1H-isoindol-1,3-dione

5'-azido-5'-deoxy-2',3'-O-isopropylidene adenosine
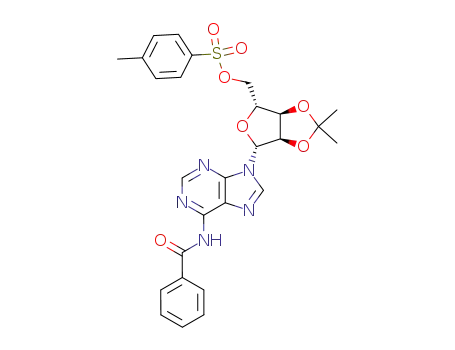
((3aR,4R,6R,6aR)-6-(6-benzamido-9H-purin-9-yl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methyl 4-methylbenzenesulfonate
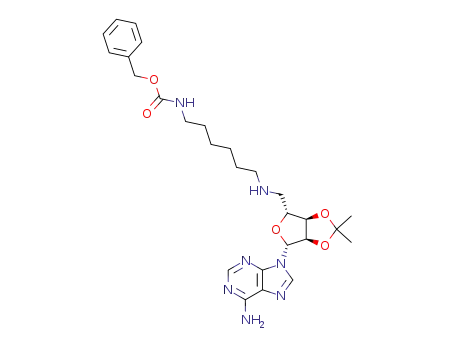
5'-<<6-<<(phenylmethoxy)carbonyl>amino>-1-hexyl>amino>-5'-deoxy-2',3'-O-isopropylideneadenosine
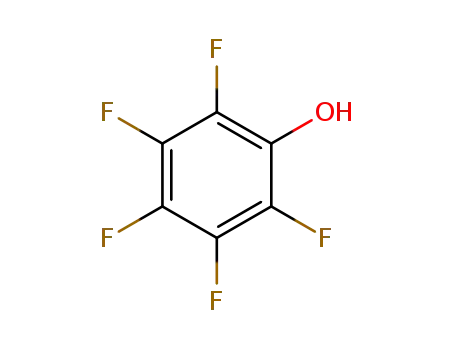
2,3,4,5,6-pentafluorophenol
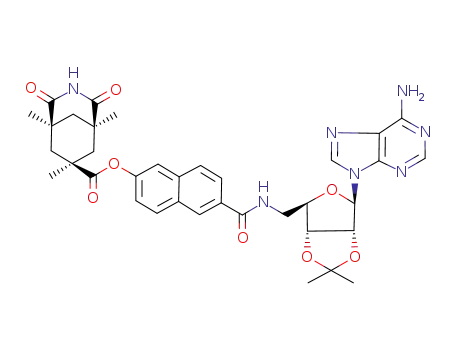
(1S,5R,7S)-1,5,7-Trimethyl-2,4-dioxo-3-aza-bicyclo[3.3.1]nonane-7-carboxylic acid 6-{[(3aR,4R,6R,6aR)-6-(6-amino-purin-9-yl)-2,2-dimethyl-tetrahydro-furo[3,4-d][1,3]dioxol-4-ylmethyl]-carbamoyl}-naphthalen-2-yl ester
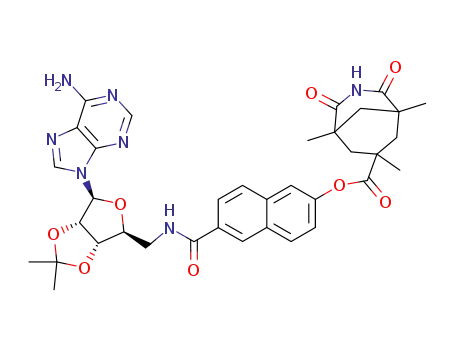
1,5,7-Trimethyl-2,4-dioxo-3-aza-bicyclo[3.3.1]nonane-7-carboxylic acid 6-{[(3aS,4S,6S,6aS)-6-(6-amino-purin-9-yl)-2,2-dimethyl-tetrahydro-furo[3,4-d][1,3]dioxol-4-ylmethyl]-carbamoyl}-naphthalen-2-yl ester