- +86 185 8909 5435
- sales@senovaph.com
Your Location:Home >Products >Organic Chemistry >25501-32-0

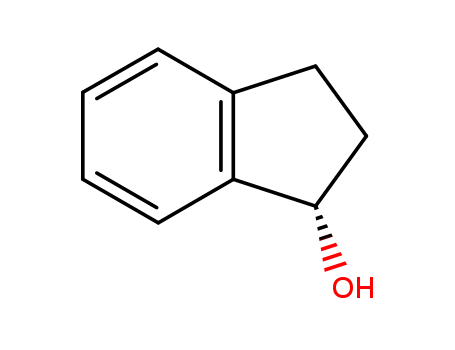
Purity:99%
|
Uses |
(S)-(+)-1-Indanol is a building block used in pharmaceutical synthesis such as orally bioavailable GPR40 agonists such as DS-1558 used to stimulate insulin secretion. |
InChI:InChI=1/C9H10O/c10-9-6-5-7-3-1-2-4-8(7)9/h1-4,9-10H,5-6H2/t9-/m0/s1
The rhodium and iridium (η5-C5Me5)MCl co...
Reduction of arylketones with a Complex ...
We report an OmpF loop deletion mutant, ...
A nonenzymatic dynamic kinetic resolutio...
It is extremely difficult to anticipate ...
A chiral cobalt pincer complex, when com...
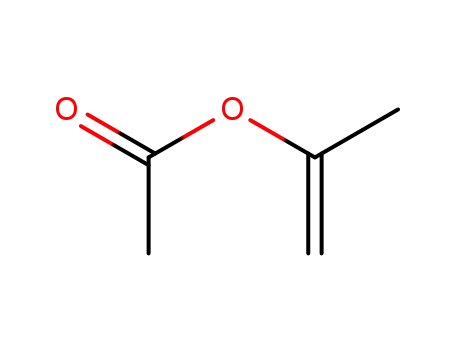
Isopropenyl acetate

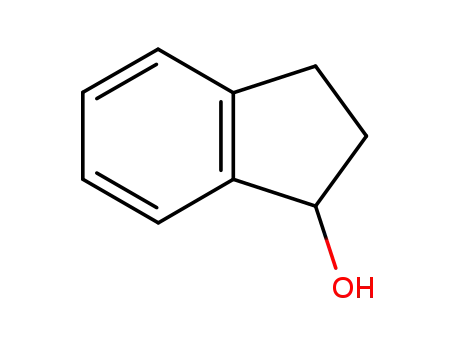
1-Indanol

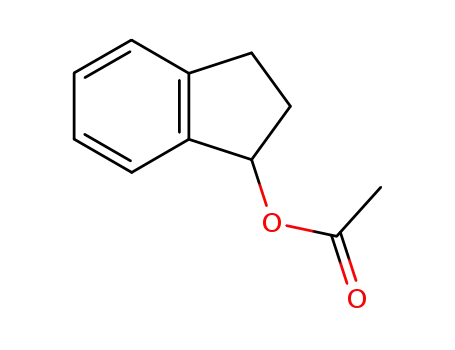
2,3-dihydro-1H-inden-1-yl-acetate

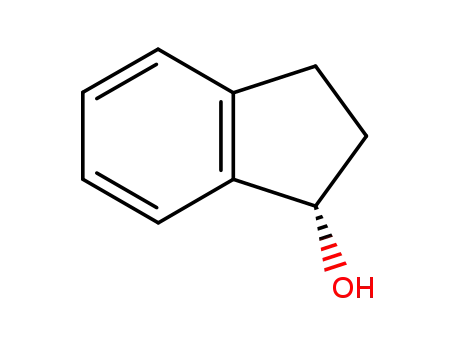
(S)-indanol
| Conditions | Yield |
|---|---|
|
With lipase from Pseudomonas cepacia immobilized on ceramic; In toluene; at 40 ℃; for 4h;
|

1-Indanol


(S)-indanol
| Conditions | Yield |
|---|---|
|
With dichloro(norbornadiene)palladium(II); oxygen; (-)-sparteine; 3 A molecular sieve; In toluene; at 60 ℃; for 54h; under 760.051 Torr;
|
25% |
|
|
|
|
Multi-step reaction with 3 steps
1: 78 percent / 4-DMAP; pyridine / tetrahydrofuran / 2 h / 20 °C
2: Candida antarctica B lipase; n-butanol / acetonitrile / 192 h
3: aq. LiOH / methanol / 3 h / Heating
With pyridine; dmap; lithium hydroxide; Candida antarctica B lipase; butan-1-ol; In tetrahydrofuran; methanol; acetonitrile;
|
|
|
Multi-step reaction with 2 steps
1: t-BuOK / (CyRuCl2)2; (1R,2S)-(+)-cis-1-aminoindan-2-ol / propan-2-ol; acetone / 1 h / 25 °C
2: t-BuOK / (CyOsCl2)2; (1R,2S)-(+)-cis-1-aminoindan-2-ol / propan-2-ol / 48 h / -24 °C
With potassium tert-butylate; (CyOsCl2)2; (CyRuCl2)2; (1R,2S)-1-Amino-2-indanol; In isopropyl alcohol; acetone;
|
|
|
Multi-step reaction with 3 steps
1: Py
2: cinchonidine
3: aq. KOH / methanol
With pyridine; potassium hydroxide; Cinchonidin; In methanol;
|
|
|
Multi-step reaction with 2 steps
1: Py
2: aq. KOH / methanol
With pyridine; potassium hydroxide; In methanol;
|
|
|
Multi-step reaction with 2 steps
1: dmap / dichloromethane / 4 h / 20 °C
2: Candida rugosa lipase / tetrahydrofuran; aq. phosphate buffer / 24 h / 30 °C / pH 7 / Resolution of racemate; Enzymatic reaction
With dmap; Candida rugosa lipase; In tetrahydrofuran; aq. phosphate buffer; dichloromethane;
|
|
|
Multi-step reaction with 2 steps
1: (R,R)-N-(1-(β-1-naphthyl)ethyl)benzoguanidine; N-ethyl-N,N-diisopropylamine / toluene / 24 h / -78 °C / Inert atmosphere
2: tetrabutyl ammonium fluoride / tetrahydrofuran / 2 h / 20 °C / Inert atmosphere
With (R,R)-N-(1-(β-1-naphthyl)ethyl)benzoguanidine; tetrabutyl ammonium fluoride; N-ethyl-N,N-diisopropylamine; In tetrahydrofuran; toluene;
|
|
|
Multi-step reaction with 2 steps
1: N-ethyl-N,N-diisopropylamine; C23H21N3 / toluene / 24 h / -78 °C / Inert atmosphere
2: tetrabutyl ammonium fluoride / tetrahydrofuran / 2 h / 20 °C / Inert atmosphere
With C23H21N3; tetrabutyl ammonium fluoride; N-ethyl-N,N-diisopropylamine; In tetrahydrofuran; toluene;
|
|
|
With 6C26H22N2O6(2-)*3C10H8N2*8Zn(2+)*2O(2-); In acetone; at 20 ℃; enantioselective reaction;
|
99.3 % ee |
|
Multi-step reaction with 2 steps
1: oxygen; dipropylene glycol dimethyl ether / 120 °C
2: C31H36N2O2RuS; sodium formate / methanol; water / 12 h / 50 °C / Inert atmosphere
With dipropylene glycol dimethyl ether; oxygen; sodium formate; C31H36N2O2RuS; In methanol; water;
|
|
|
Multi-step reaction with 2 steps
1: (OC-6-23)-[2-[6-[(amino-κN)methyl]-2-pyridinyl-κN]-5-methylphenyl-κC][1,1'-(1,4-butanediyl)bis[1,1-diphenylphosphine-κP]]chlororuthenium(II); copper(l) chloride; sodium t-butanolate; (R,R)-1,2-bis(2,5-diphenylphospholanyl)ethane / toluene / 14 h / 20 °C / Glovebox; Inert atmosphere
2: tetrabutyl ammonium fluoride / tetrahydrofuran / 0.5 h / 20 °C / Inert atmosphere
With (OC-6-23)-[2-[6-[(amino-κN)methyl]-2-pyridinyl-κN]-5-methylphenyl-κC][1,1'-(1,4-butanediyl)bis[1,1-diphenylphosphine-κP]]chlororuthenium(II); tetrabutyl ammonium fluoride; copper(l) chloride; sodium t-butanolate; (R,R)-1,2-bis(2,5-diphenylphospholanyl)ethane; In tetrahydrofuran; toluene;
|
|
|
1-Indanol; With (Δ)12-PCC-57; In acetone; at 20 ℃; for 8h;
In dichloromethane; enantioselective reaction;
|
99.7 % ee |
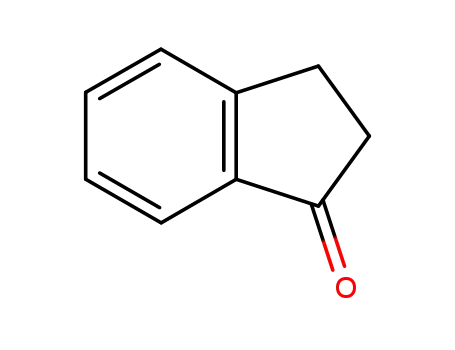
inden-1-one

Isopropenyl acetate

1-Indanol
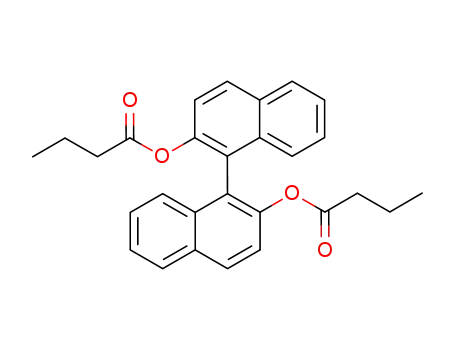
<1,1'-Binaphthalene>-2,2'-diol dibutanoate
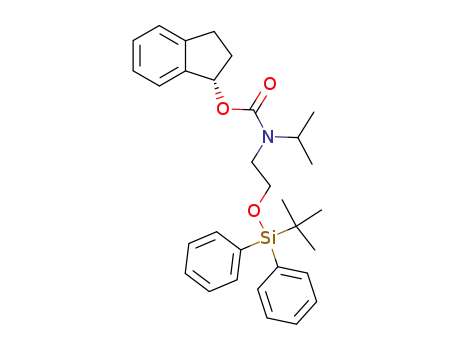
[2-(tert-Butyl-diphenyl-silanyloxy)-ethyl]-isopropyl-carbamic acid (S)-indan-1-yl ester

inden-1-one
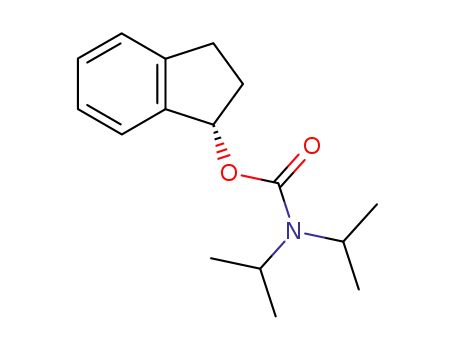
(S)-N,N-diisopropyl O-(indan-1-yl)carbamate
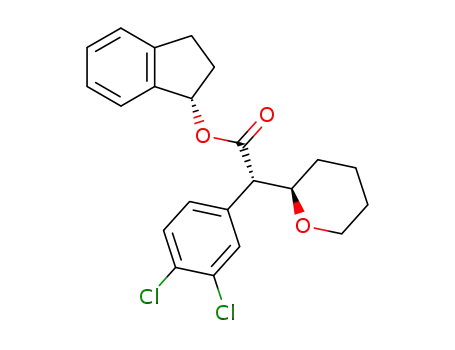
(2S,2R')-(3,4-dichlorophenyl)(tetrahydropyran-2-yl)acetic acid indanyl ester