- +86 185 8909 5435
- sales@senovaph.com
Your Location:Home >Products >Organic Chemistry >127641-25-2

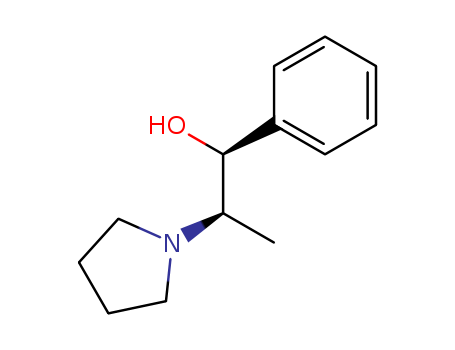
Purity:99%
|
Uses |
(1R,2S)-2-Pyrrolidino-1-phenyl-1-propanol, is a chiral building block used in asymmetric synthesis. It is also used as a novel heterodimer chiral amide base for enantioselective deprotonation of ketones. |
InChI:InChI=1/C13H19NO/c1-11(14-9-5-6-10-14)13(15)12-7-3-2-4-8-12/h2-4,7-8,11,13,15H,5-6,9-10H2,1H3/t11-,13-/m0/s1
We report herein an enantioselective con...
The invention belongs to the technical f...
The present invention relates to a proce...
The invention provides a new method for ...
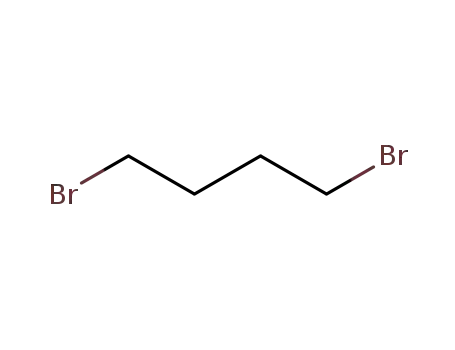
1,4-dibromo-butane

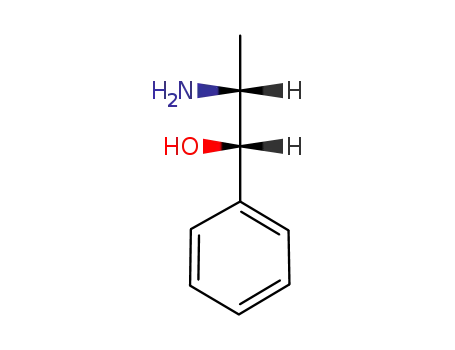
(1S,2R)-(+)-norphedrine

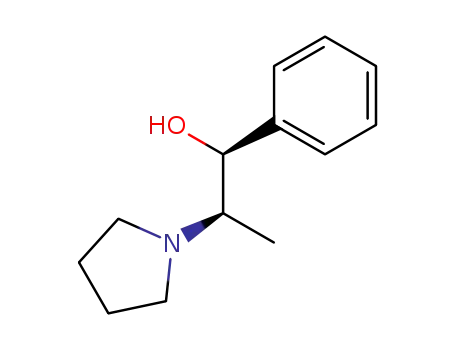
(1S,2R)-(+)-1-phenyl-2-(1-pyrrolidinyl)-1-propanol
| Conditions | Yield |
|---|---|
|
With sodium hydrogencarbonate; In toluene; at 105 - 115 ℃; for 24h; Temperature; Dean-Stark; Inert atmosphere; Large scale;
|
87% |
|
With sodium hydrogencarbonate; In toluene; for 48h; Inert atmosphere; Reflux;
|
82% |
|
With tetra-(n-butyl)ammonium iodide; sodium carbonate; In tetrahydrofuran; for 40h; Heating;
|
|
|
With tetra-(n-butyl)ammonium iodide; sodium carbonate; In tetrahydrofuran; for 48h; Heating;
|
|
|
With sodium carbonate;
|
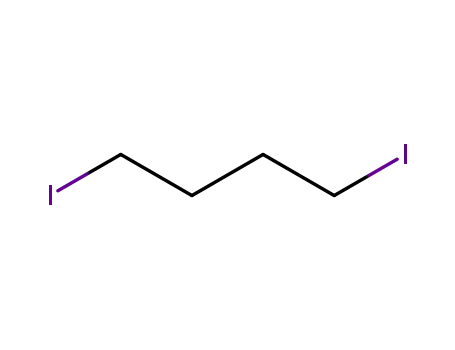
1,4-Diiodobutane


(1S,2R)-(+)-norphedrine


(1S,2R)-(+)-1-phenyl-2-(1-pyrrolidinyl)-1-propanol
| Conditions | Yield |
|---|---|
|
With potassium carbonate; In ethanol; Heating;
|
33% |

1,4-Diiodobutane

(1S,2R)-(+)-norphedrine

1,4-dibromo-butane
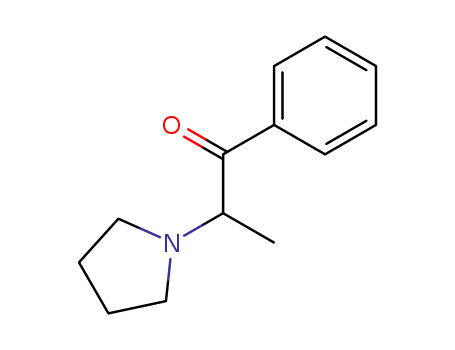
(SR)-(+/-)-1-phenyl-2-(1-pyrrolidinyl)-1-propanone
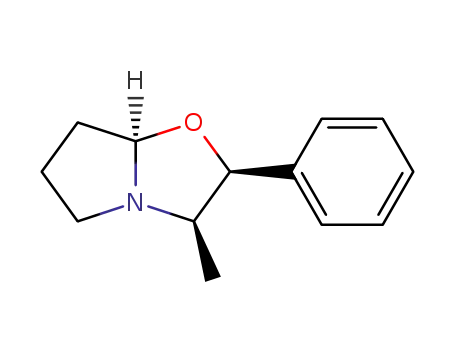
(2S,3R,7aR)-3-methyl-2-phenylhexahydropyrrolo[2.1-b]oxazole
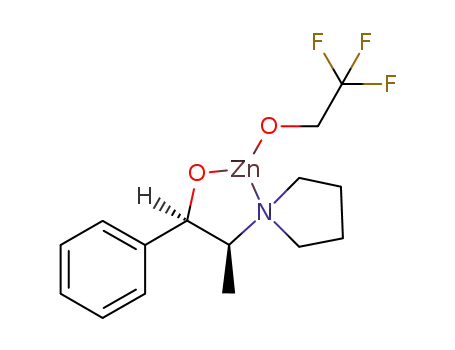
C15H20F3NO2Zn
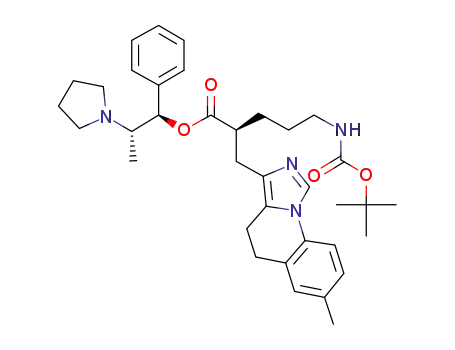
(1R,2S)-1-phenyl-2-(pyrrolidin-1-yl)propyl (2S)-5-[(tert-butoxycarbonyl)amino]-2-[(7-methyl-4,5-dihydroimidazo[1,5-a]quinolin-3-yl)methyl]pentanoate
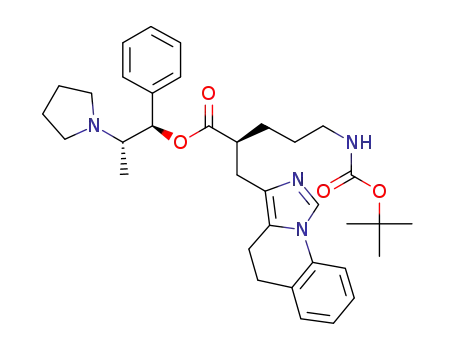
(1R,2S)-1-phenyl-2-(pyrrolidin-1-yl)propyl (2S)-5-[(tert-butoxycarbonyl)amino]-2-(4,5-dihydroimidazo[1,5-a]quinolin-3-ylmethyl)pentanoate