SENOVA PHARMA
- +86 185 8909 5435
- sales@senovaph.com
Your Location:Home >Products >Organic Chemistry >7287-82-3

Purity:99%
(Chemical Equation Presented) The effect...
We have prepared and characterized two r...
Most ligands applied for asymmetric hydr...
The enantioselective hydroboration of vi...
I-interacting ligands of the diphosphino...
The asymmetric transfer hydrogenation (A...
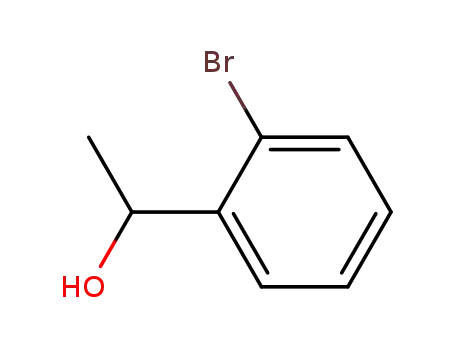
1-(2-bromophenyl)ethanol

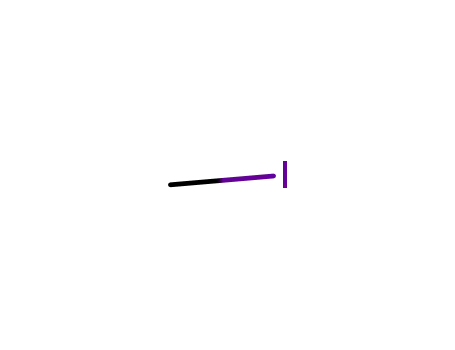
methyl iodide

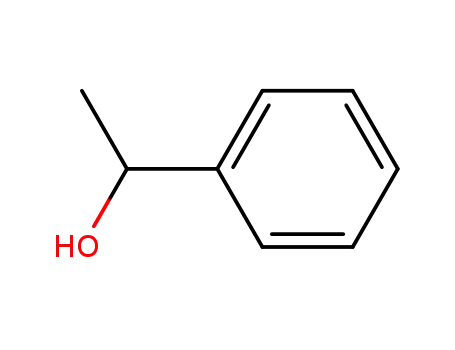
1-Phenylethanol

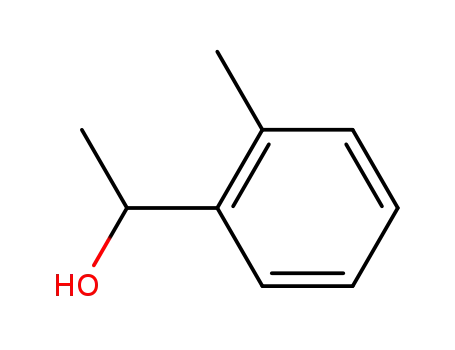
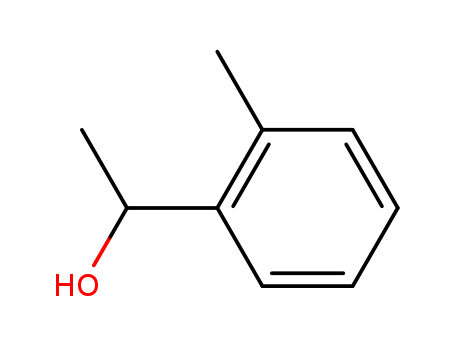
1-O-tolyl-ethanol
| Conditions | Yield |
|---|---|
|
1-(2-bromophenyl)ethanol; With diisopropylmagnesium; lithium 2-(dimethylamino)ethanolate; In diethyl ether; at -40 - 20 ℃; Inert atmosphere;
methyl iodide; With copper(I) cyanide di(lithium chloride); In tetrahydrofuran; at 0 - 20 ℃; Inert atmosphere;
|
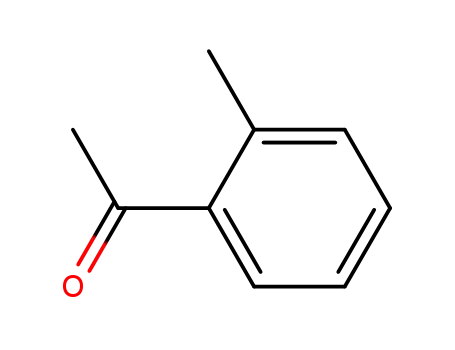
2-Methylacetophenone

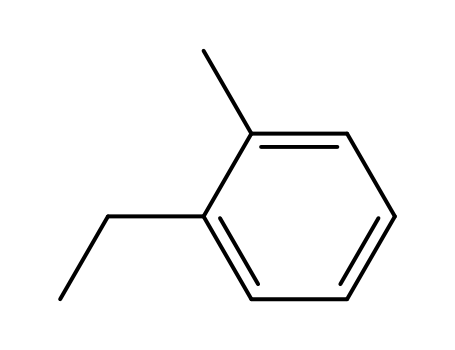
2-Ethyltoluene

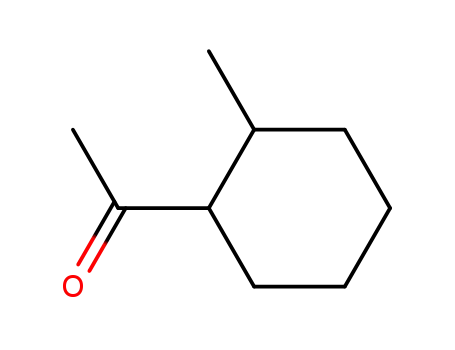
1-(2-methylcyclohexyl)ethanone


1-O-tolyl-ethanol
| Conditions | Yield |
|---|---|
|
With Rh*C4H8O*C18H15P; hydrogen; In tetrahydrofuran; at 30 ℃; for 5h; under 15001.5 Torr; Autoclave; Glovebox;
|
24 %Chromat. 59 %Chromat. 14 %Chromat. |
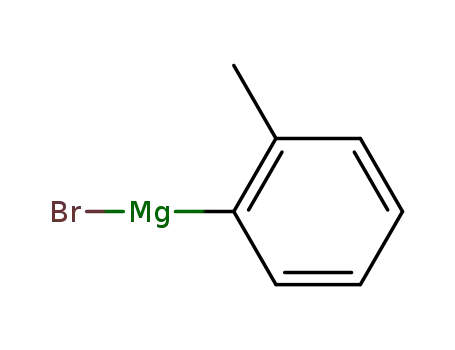
ortho-tolylmagnesium bromide
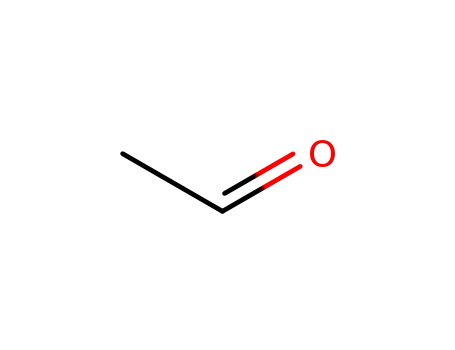
acetaldehyde
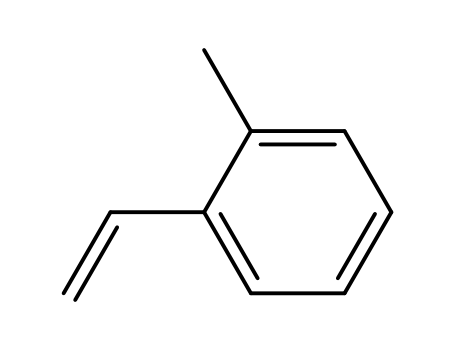
1-methyl-2-vinyl-benzene
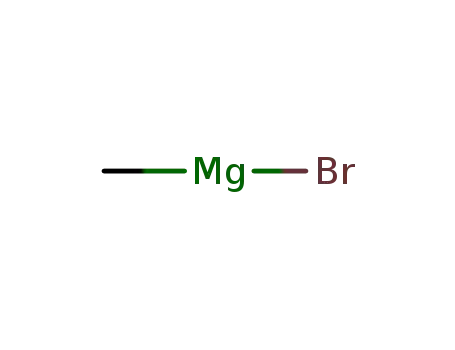
methylmagnesium bromide

1-methyl-2-vinyl-benzene

2-Methylacetophenone
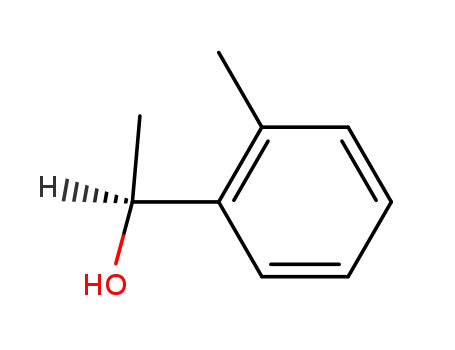
(S)-1-(2-Methylphenyl)ethanol
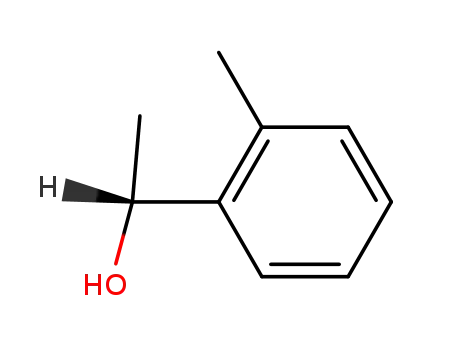
(R)-1-(2-methylphenyl)ethanol