SENOVA PHARMA
- +86 185 8909 5435
- sales@senovaph.com
Your Location:Home >Products >Active intermediate >188770-83-4
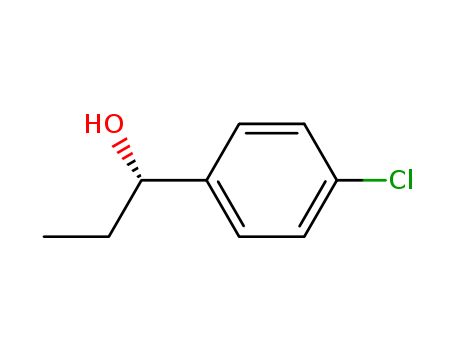

Purity:99%
(S)1-(3-NITROPHENYL)PROPANOL can used as API.
InChI:InChI=1/C9H11NO3/c1-2-9(11)7-4-3-5-8(6-7)10(12)13/h3-6,9,11H,2H2,1H3/t9-/m0/s1
The substitution of tropos 2,2′-biphenol...
Several novel chiral sulfonamide ligands...
In the presence of PhSiH3 as the hydride...
We present the synthesis of β-hydroxy su...
diethylzinc

3-nitro-benzaldehyde

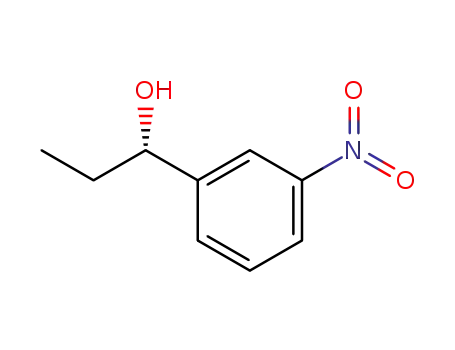
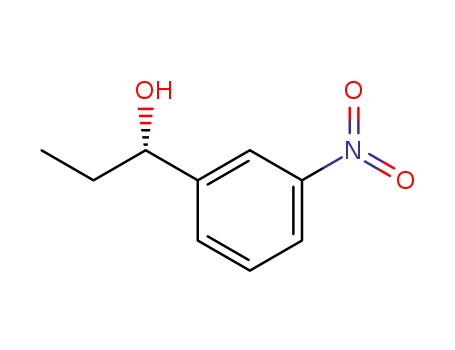
(S)-1-(3-nitrophenyl)propan-1-ol
| Conditions | Yield |
|---|---|
|
chiral polymer-supported Ti-complex of substituted R-BINOL; In dichloromethane; at 0 ℃; for 54h;
|
88% |
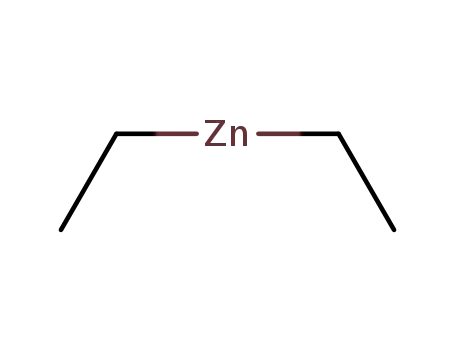
diethylzinc


3-nitro-benzaldehyde

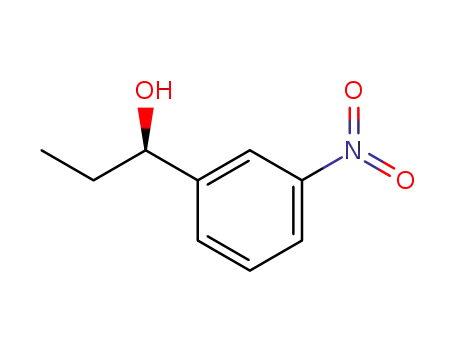
(R)-1-(3-nitrophenyl)propan-1-ol


(S)-1-(3-nitrophenyl)propan-1-ol
| Conditions | Yield |
|---|---|
|
With (S)-2,2'-dihydroxy-1,1'-binaphthyl; titanium(IV) isopropylate; In hexane; dichloromethane; at 0 ℃; for 5h; Yield given. Yields of byproduct given. Title compound not separated from byproducts;
|
|
|
With titanium-(S)-5,5',6,6',7,7',8,8'-octahydro-1,1'-bi-2-naphthol; Multistep reaction. Title compound not separated from byproducts; 1.) CH2Cl2, hexane, RT, 10 min, 2.) CH2Cl2, hexane, 0 deg C, 5 h;
|
|
|
chiral polymer-supported Ti-complex of substituted R-BINOL; In dichloromethane; at 0 ℃; for 50h; Title compound not separated from byproducts;
|
|
|
With (1R,2R)-1,2-diphenylethylenediamine-based polymer; In toluene; at -40 - 30 ℃; Title compound not separated from byproducts.;
|
|
|
With chiral N,N-bis(2-hydroxybenzyl)amine-derived ligand; In hexane; toluene; at 20 ℃; for 3h; Title compound not separated from byproducts.;
|
|
|
With titanium(IV) isopropylate; methyl 4,6-O-benzylidene-2-deoxy-2-trifluoromethylsulfonamido-α-D-glucopyranoside; In dichloromethane; toluene; at 20 ℃; optical yield given as %ee; enantioselective reaction; Inert atmosphere;
|
|
|
diethylzinc; With (1R,2S,4R,5S)-1,4-N,N-bis(p-methylphenylsulfonamino)-2,5-dimethylcyclohexane; In hexane; toluene; at 20 ℃; for 0.333333h; Inert atmosphere;
With titanium(IV) isopropylate; In hexane; toluene; at -78 ℃; for 0.5h; Inert atmosphere;
3-nitro-benzaldehyde; In hexane; toluene; at -25 ℃; for 24h; optical yield given as %ee; enantioselective reaction; Inert atmosphere;
|
|
|
With titanium(IV) isopropylate; C42H50N4O8; In dichloromethane; toluene; at 0 ℃; for 16h; enantioselective reaction; Schlenk technique; Inert atmosphere;
|
49.8 % ee |
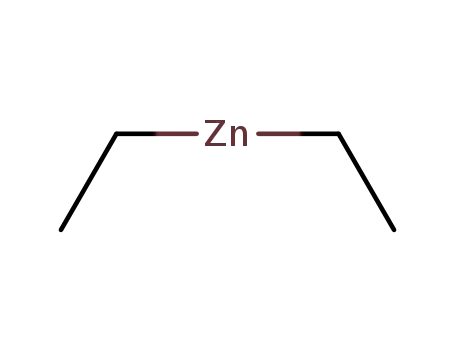
diethylzinc

3-nitro-benzaldehyde
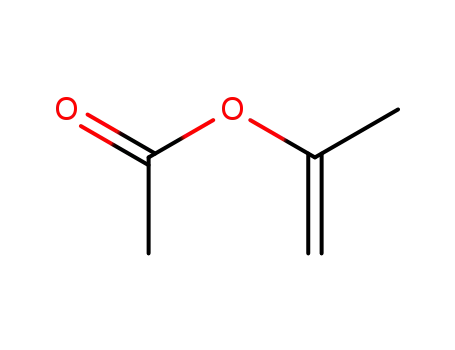
Isopropenyl acetate
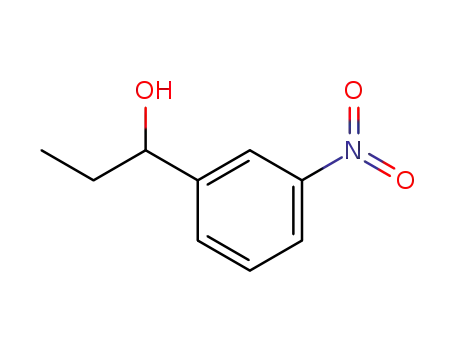
α-ethyl-3-nitrobenzyl alcohol
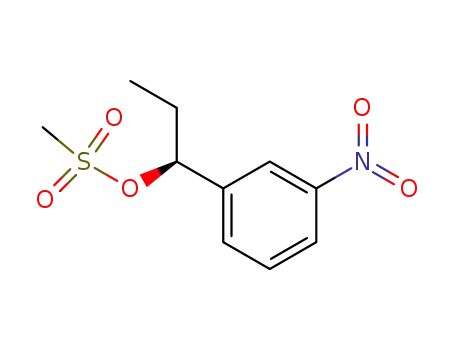
(S)-1-(3-nitrophenyl)propanol mesylate
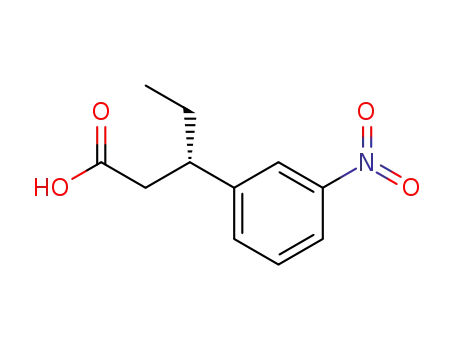
(S)-3-(3-nitrophenyl)pentanoic Acid