- +86 185 8909 5435
- sales@senovaph.com
Your Location:Home >Products >Pharmaceutical intermediates >76116-20-6

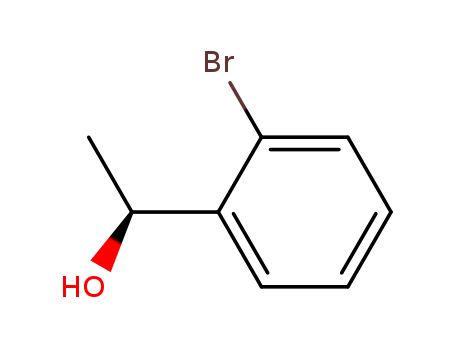
Purity:99%
|
Chemical Properties |
white to light yellow crystal powder |
|
Uses |
It is the most important dyestuff intermediate. |
InChI:InChI=1/C8H9BrO/c1-6(10)7-4-2-3-5-8(7)9/h2-6,10H,1H3/t6-/m1/s1
Most ligands applied for asymmetric hydr...
The asymmetric transfer hydrogenation (A...
A nonenzymatic dynamic kinetic resolutio...
The Noyori-Ikariya (arene)Ru(II)/TsDPEN ...
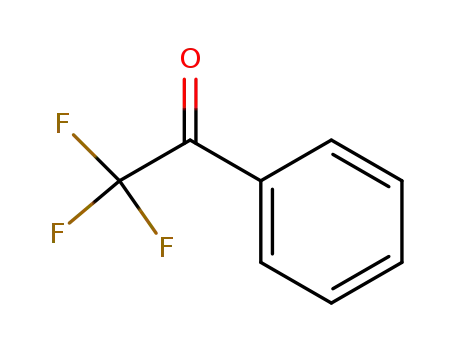
2,2,2-Trifluoroacetophenone

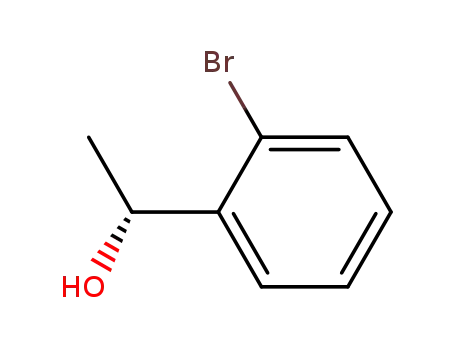
(1R)-1-(2-bromophenyl)ethan-1-ol

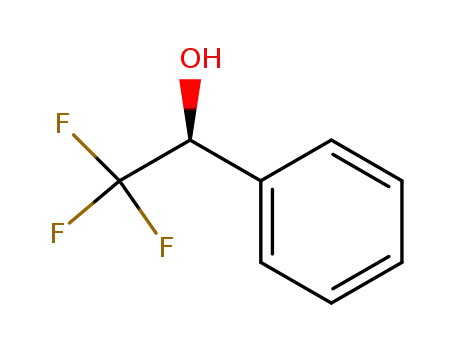
S(+)-1-phenyl-2,2,2-trifluoroethanol
| Conditions | Yield |
|---|---|
|
With ethanol; NADH; In aq. buffer; at 35 ℃; for 15h; pH=7; stereoselective reaction; Microbiological reaction; Enzymatic reaction;
|
32.3 % ee |
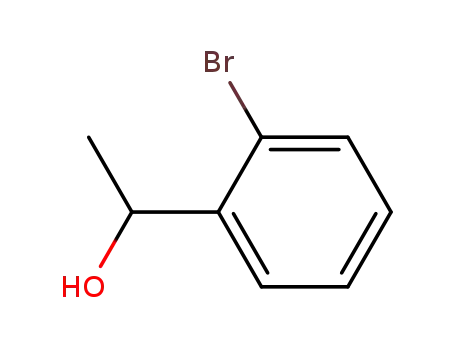
1-(2-bromophenyl)ethanol

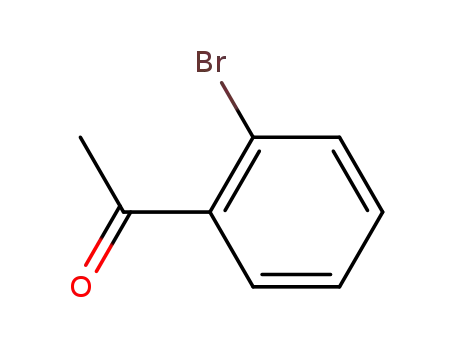
2-bromophenyl methyl ketone

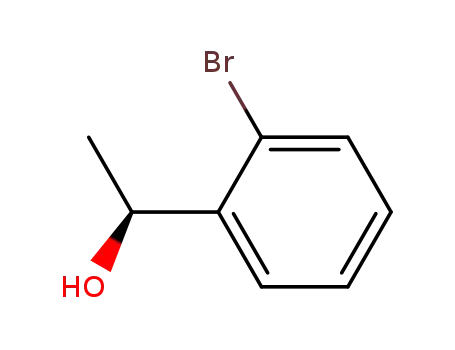
(1S)-1-(2-bromophenyl)ethanol


(1R)-1-(2-bromophenyl)ethan-1-ol
| Conditions | Yield |
|---|---|
|
With sodium hydrogencarbonate; sodium bromide; chiral 4,1'-dinaphthyl-3,2'-cyclo[C(Me)2-N(oxyl)-C(Me)2]; In dichloromethane; water; at -15 ℃; for 1.5h; Title compound not separated from byproducts; Electrolysis;
|
54% |
|
1-(2-bromophenyl)ethanol; With N,N'-bis(salicylidene)-1,2-cyclohexanediaminomanganese(III) chloride; potassium acetate; In dichloromethane; water; for 0.0833333h;
With N-Bromosuccinimide; In dichloromethane; water; at 20 ℃; for 4h; enantioselective reaction; Kinetics;
|
85 % ee |

2-bromophenyl methyl ketone

1-(2-bromophenyl)ethanol

dimethyl zinc(II)
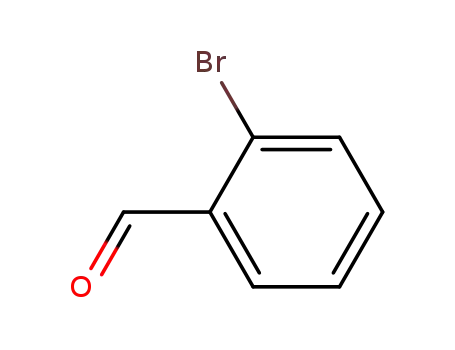
ortho-bromobenzaldehyde
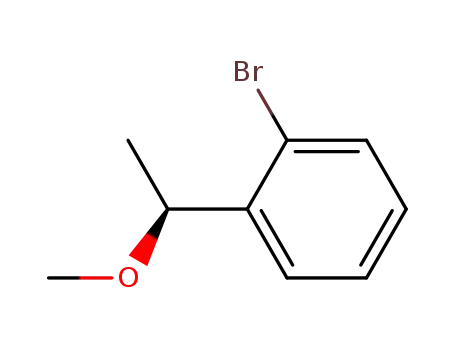
(S)-1-bromo-2-(1-methoxyethyl)benzene
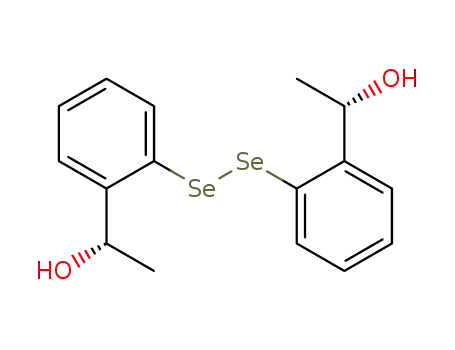
(S,S)-bis[2-(1-hydroxyethyl)phenyl] diselenide
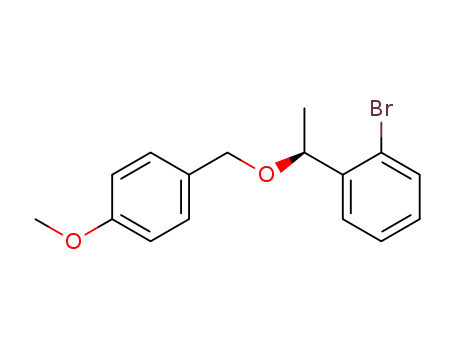
C16H17BrO2
(S)-[1-(2-bromophenyl)ethoxy]-tert-butyldimethylsilane