- +86 185 8909 5435
- sales@senovaph.com
Your Location:Home >Products >Organic Chemistry >1865-56-1

Purity:99%
The Vilsmeier-Haack reaction of 3β-aceto...
An approach to steroids annulated with p...
In our effort to discover potent and spe...
Polyhydroxy steroids bearing the 3β,5α,6...
The invention relates to the field of me...
A process for the synthesis of 3β-hydrox...
A general, practical, and simple synthes...
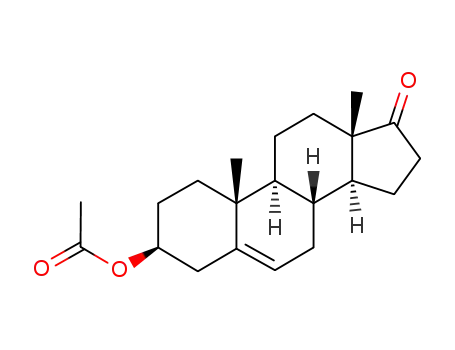
prasterone acetate

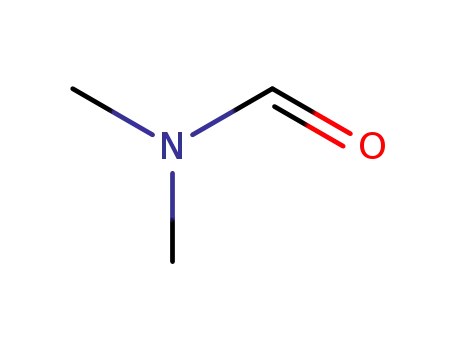
N,N-dimethyl-formamide

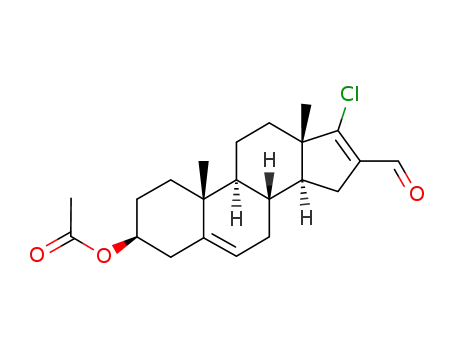
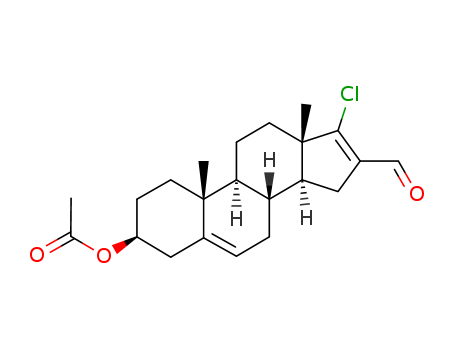
3β-acetoxy-17-chloro-16-formylandrost-5,16-diene
| Conditions | Yield |
|---|---|
|
With trichlorophosphate; In chloroform; for 5h; Reflux; Inert atmosphere;
|
82% |
|
With trichlorophosphate; In chloroform; for 5h; Cooling with ice; Reflux; Inert atmosphere;
|
78.7% |
|
With trichlorophosphate; at 80 ℃; for 5h; Inert atmosphere;
|
77% |
|
With trichlorophosphate; at 80 ℃; for 2h;
|
77.49% |
|
N,N-dimethyl-formamide; With trichlorophosphate; In chloroform; at 20 ℃; for 0.666667h; Cooling with ice;
prasterone acetate; In chloroform; for 3h; Reflux;
|
75% |
|
With trichlorophosphate; In chloroform;
|
74% |
|
With trichlorophosphate; at 55 - 65 ℃; for 3h;
|
65% |
|
With trichlorophosphate; at 65 ℃; for 3h;
|
65% |
|
With trichlorophosphate; In chloroform; for 4h; Heating;
|
62% |
|
With trichlorophosphate;
|
|
|
With trichlorophosphate; In chloroform; Reflux; Inert atmosphere;
|
|
|
With trichlorophosphate; In chloroform; at 80 ℃;
|
|
|
With trichlorophosphate; In chloroform; Inert atmosphere; Reflux;
|
|
|
With trichlorophosphate; In chloroform; for 5h; Inert atmosphere; Reflux;
|
|
|
With trichlorophosphate; In chloroform; Reflux; Inert atmosphere;
|
|
|
|
|
|
With trichlorophosphate; In chloroform; for 5h; Reflux;
|
|
|
N,N-dimethyl-formamide; With trichlorophosphate; at 0 - 20 ℃; Inert atmosphere;
prasterone acetate; In N,N-dimethyl-formamide; at 20 - 75 ℃; for 2.91667h; Inert atmosphere;
|
|
|
With trichlorophosphate; at 55 - 65 ℃; for 3h;
|

prasterone acetate


N,N-dimethyl-formamide


3β-acetoxy-17-chloro-16-formylandrost-5,16-diene

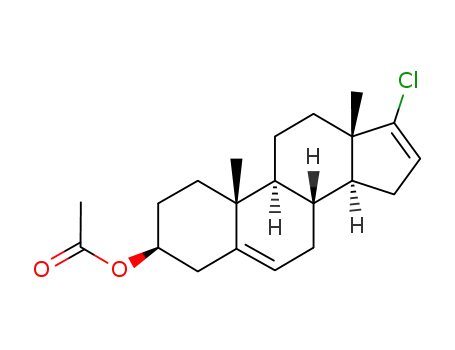
17-chlorroandrosta-5,16-dien-3β-yl acetate
| Conditions | Yield |
|---|---|
|
With trichlorophosphate; In chloroform; for 5h; Heating;
|
11.4% 77% |
|
With trichlorophosphate; In various solvent(s); Heating;
|
|
|
With trichlorophosphate; In chloroform; Heating / reflux;
|
|
|
With trichlorophosphate; In chloroform; Reflux;
|
|
|
With trichlorophosphate; In chloroform; at 85 ℃; Inert atmosphere;
|

prasterone acetate

N,N-dimethyl-formamide
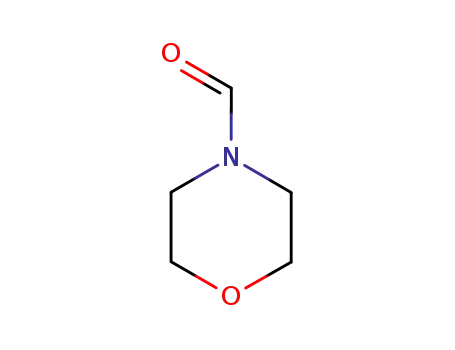
4-morpholinecarboxaldehyde
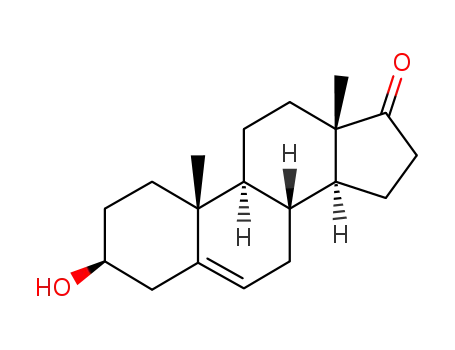
dehydroepiandrosterone
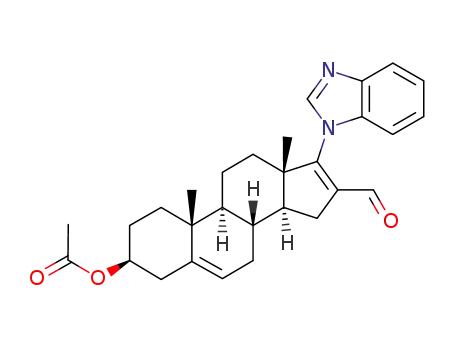
3-β-acetoxy-17-(1H-benzimidazol-1-yl)-16-formyl-androsta-5,16-diene
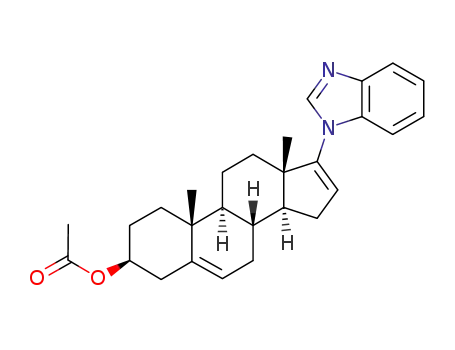
3β-acetoxy-17-(1H-benzimidazol-1-yl)-androsta-5,16-diene
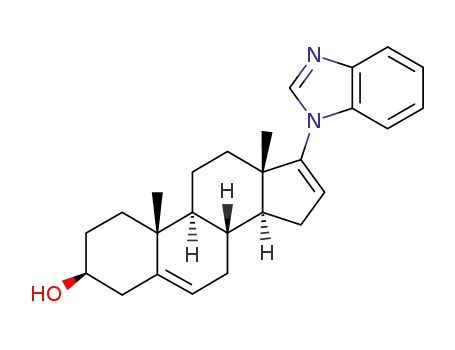
Galeterone
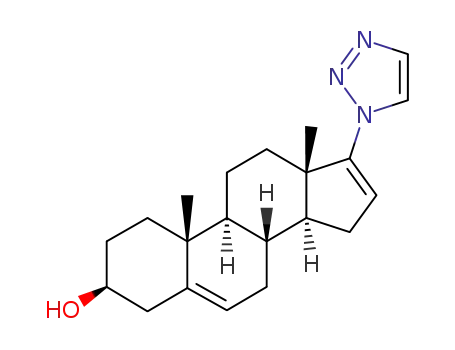
3β-hydroxy-17-(1H-1,2,3-triazol-1-yl)androsta-5,16-diene