SENOVA PHARMA
- +86 185 8909 5435
- sales@senovaph.com
Your Location:Home >Products >Pharmaceutical intermediates >73890-73-0
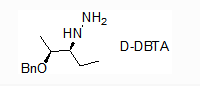

Purity:99%
We have demonstrated a green chemistry a...
Manganese catalysts derived from trident...
This manuscript describes the design and...
The synthesis and resolution of new trid...
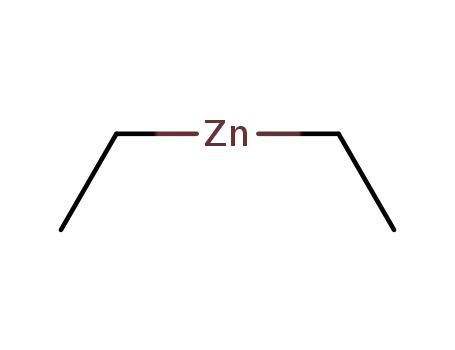
diethylzinc

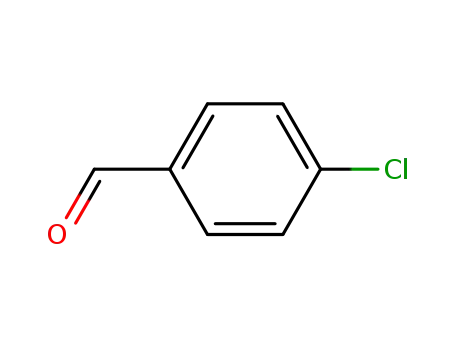
4-chlorobenzaldehyde

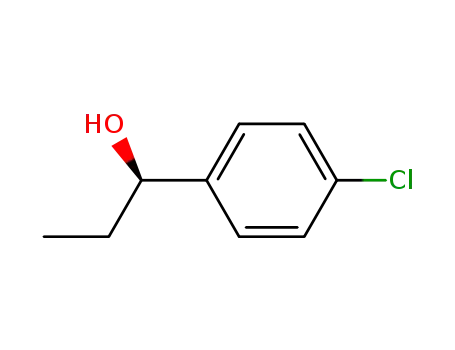
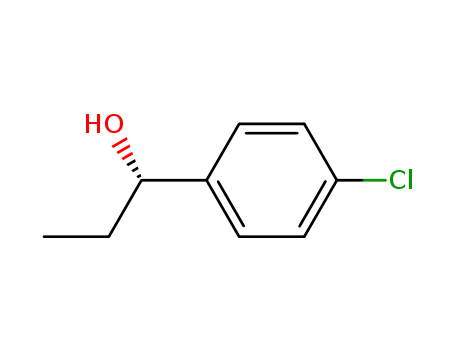
(R)-1-(4-chlorophenyl)-1-propanol

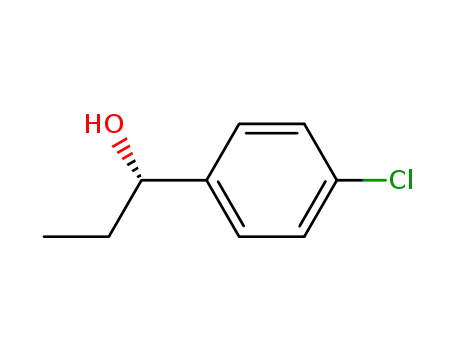
(S)-1-(4-chlorophenyl)-1-propanol

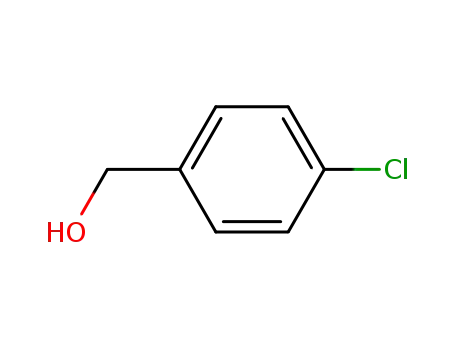
para-Chlorobenzyl alcohol
| Conditions | Yield |
|---|---|
|
With (1S)-(+)-3-exo-(dimethylamino)isoborneol; In toluene; at 0 ℃; for 12h; Yields of byproduct given. Title compound not separated from byproducts;
|
2% 86% |
|
With polymer-supported camphor derivative; In toluene; at 20 ℃; Title compound not separated from byproducts;
|
13% |
|
With (P,P)-(+)-bis[5]helicene diol; In toluene; for 24h; Title compound not separated from byproducts;
|
|
|
With chiral bicyclo[3.2.1]octane-based alcohol; In hexane; toluene; at 20 ℃; for 24h; Title compound not separated from byproducts;
|
|
|
With (-)-8-(9H-fluoren-9-ylidene)-1-(2-hydroxyphenyl)-7-methyl-5,6,7,8-tetrahydronaphthalen-2-ol; In hexane; toluene; at 0 ℃; for 168h; enantioselective reaction; Schlenk technique; Inert atmosphere;
|
35 % ee |
|
With (-)-8-(9H-fluoren-9-ylidene)-1-(2-hydroxyphenyl)-7-methyl-5,6,7,8-tetrahydronaphthalen-2-ol; In hexane; toluene; at 0 ℃; for 168h; enantioselective reaction; Schlenk technique; Inert atmosphere;
|
24 % ee |
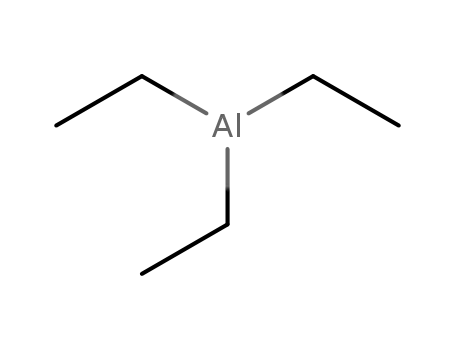
triethylaluminum


4-chlorobenzaldehyde


(R)-1-(4-chlorophenyl)-1-propanol


(S)-1-(4-chlorophenyl)-1-propanol


para-Chlorobenzyl alcohol
| Conditions | Yield |
|---|---|
|
triethylaluminum; With titanium(IV) isopropylate; L-3-phenyllactic acid; In tetrahydrofuran; toluene; at 21 ℃; for 4h;
4-chlorobenzaldehyde; In tetrahydrofuran; toluene; at 21 ℃; for 4h; Title compound not separated from byproducts;
|
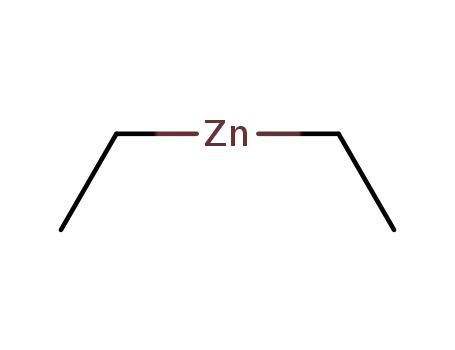
diethylzinc

4-chlorobenzaldehyde

triethylaluminum
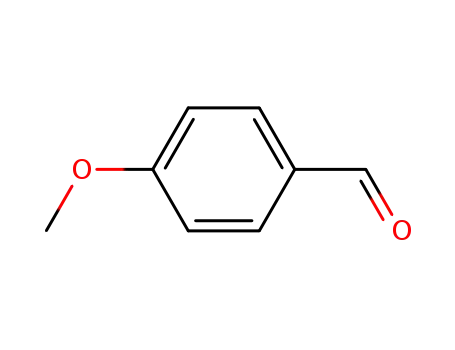
4-methoxy-benzaldehyde
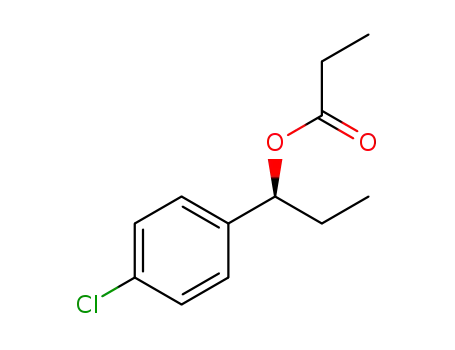
C12H15ClO2