- +86 185 8909 5435
- sales@senovaph.com
Your Location:Home >Products >Organic Chemistry >120466-66-2

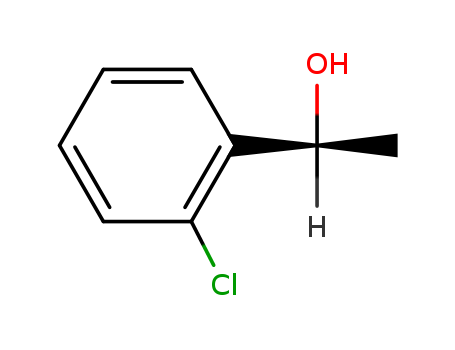
Purity:99%
A ruthenium(II) complex generated in sit...
Reduction of ketones with a reductant, 2...
The asymmetric reduction of ketones is a...
B-Chlorodiiso-2-ethylapopinocampheylbora...
Four kinds of mesoporous material-suppor...
A germinated radish sprout was used as a...
The asymmetric reduction of prochiral ke...
-
A new fluorous ligand was synthesized fr...
1,2-Disubstituted planar chiral ruthenoc...
A heterogeneous catalyst of Ir(I)-9-amin...
The oxidative kinetic resolution of race...
-
Most ligands applied for asymmetric hydr...
Cyanobacteria Synechocystis sp. PCC 6803...
Enzyme stereoselectivity control is stil...
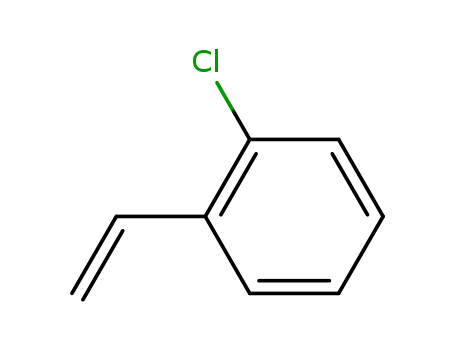
2-chlorostyrene

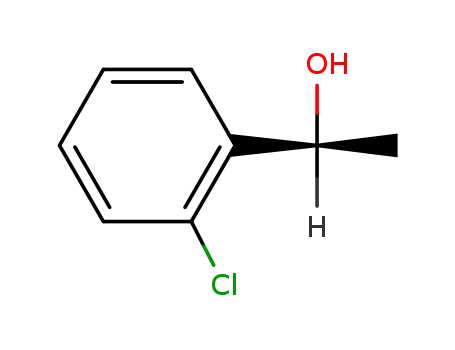
(S)-1-(2'-chlorophenyl)ethanol

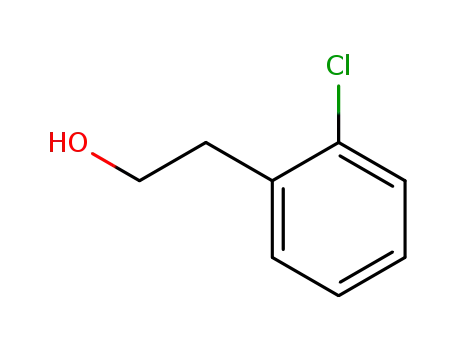
2-(2-chlorophenyl)-1-ethanol

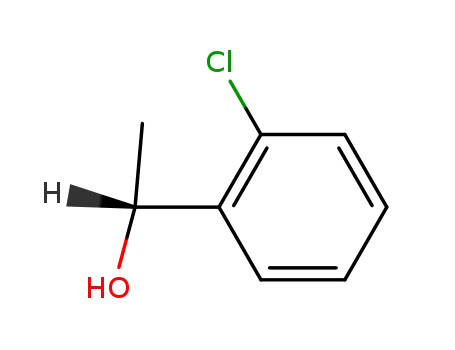
(R)-1-(o-chlorophenyl)ethanol
| Conditions | Yield |
|---|---|
|
2-chlorostyrene; With benzo[1,3,2]dioxaborole; rhodium complex 8; In toluene; at 20 ℃; for 2h;
With sodium hydroxide; water; dihydrogen peroxide; In toluene; at 20 ℃; for 2h; Title compound not separated from byproducts;
|
|
|
2-chlorostyrene; With bis(1,5-cyclooctadiene)rhodium(I) tetrafluoroborate; (1R,2R)-1,2-bis(di[2-furyl]phosphanyl)cyclohexane; benzo[1,3,2]dioxaborole; In 1,2-dimethoxyethane; toluene; at -75 ℃; for 1h;
With potassium hydroxide; dihydrogen peroxide; In 1,2-dimethoxyethane; Title compound not separated from byproducts;
|
|
|
With pinacol borane; 4 A molecular sieve; Rh(2,5-norbornadiene)2BF4; In 1,2-dimethoxyethane; at 20 ℃; for 12h; Title compound not separated from byproducts.;
|

2-chlorostyrene


(S)-1-(2'-chlorophenyl)ethanol


2-(2-chlorophenyl)-1-ethanol


(R)-1-(o-chlorophenyl)ethanol
| Conditions | Yield |
|---|---|
|
2-chlorostyrene; With benzo[1,3,2]dioxaborole; rhodium complex 8; In toluene; at 20 ℃; for 2h;
With sodium hydroxide; water; dihydrogen peroxide; In toluene; at 20 ℃; for 2h; Title compound not separated from byproducts;
|
|
|
2-chlorostyrene; With bis(1,5-cyclooctadiene)rhodium(I) tetrafluoroborate; (1R,2R)-1,2-bis(di[2-furyl]phosphanyl)cyclohexane; benzo[1,3,2]dioxaborole; In 1,2-dimethoxyethane; toluene; at -75 ℃; for 1h;
With potassium hydroxide; dihydrogen peroxide; In 1,2-dimethoxyethane; Title compound not separated from byproducts;
|
|
|
With pinacol borane; 4 A molecular sieve; Rh(2,5-norbornadiene)2BF4; In 1,2-dimethoxyethane; at 20 ℃; for 12h; Title compound not separated from byproducts.;
|
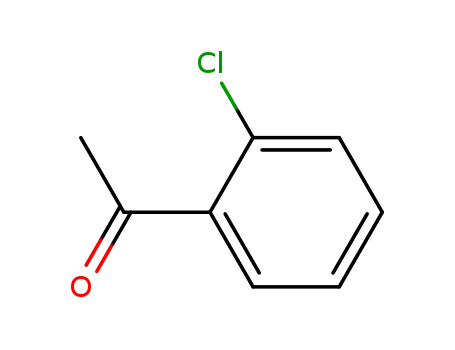
1-(2-chlorophenyl)ethanone

2-chlorostyrene
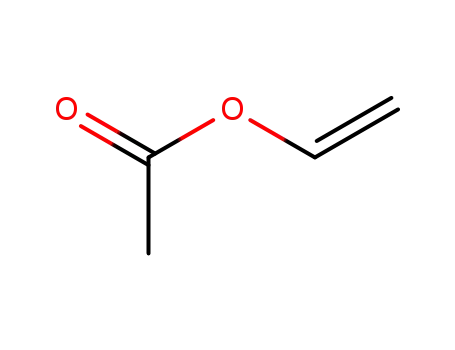
vinyl acetate
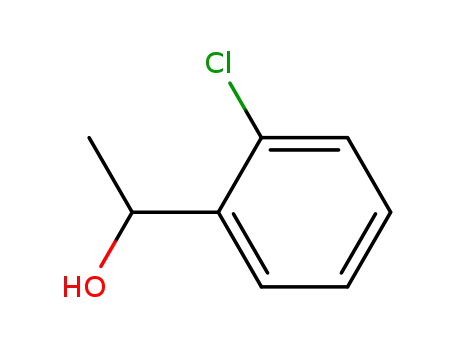
2'-chloro-sec-phenethyl alcohol
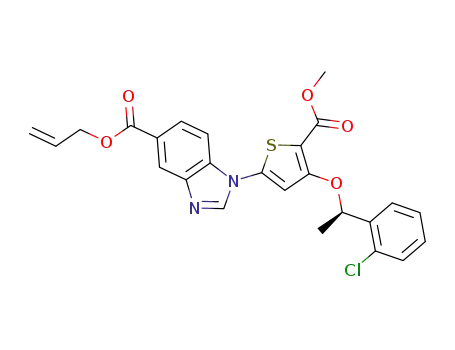
2-propen-1-yl 1-{4-{[(1R)-1-(2-chlorophenyl)ethyl]oxy}-5-[(methyloxy)carbonyl]-2-thienyl}-1H-benzimidazole-5-carboxylate
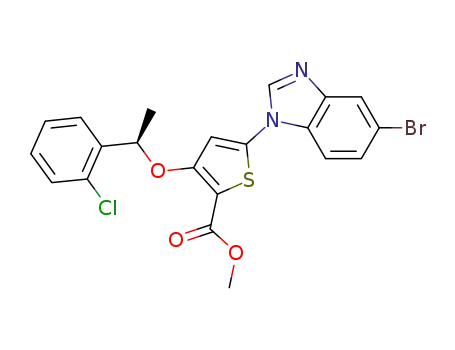
methyl 5-(5-bromo-1H-benzimidazol-1-yl)-3-{[(1R)-1-(2-chlorophenyl)ethyl]oxy}thiophene-2-carboxylate
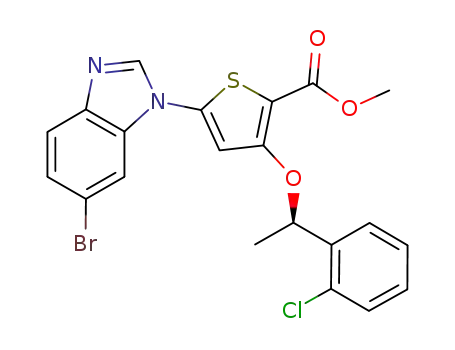
C21H16BrClN2O3S
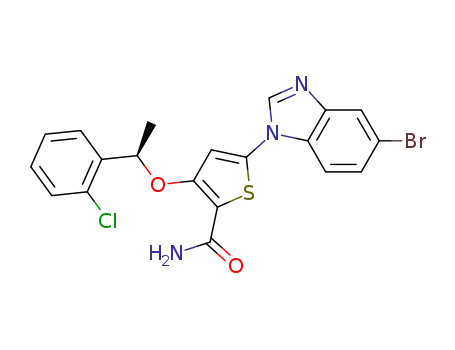
5-(5-bromo-1H-benzimidazol-1-yl)-3-({(1R)-1-[2-chlorophenyl]ethyl}oxy)thiophene-2-carboxamide