- +86 185 8909 5435
- sales@senovaph.com
Your Location:Home >Products >Analytical Chemistry >875444-08-9

Purity:99%
The crystal structure of the (4S,5R)-5-[...
The invention provides a chiral purifica...
We describe our optimization efforts to ...
The invention relates to Ana Qubo, and p...
The invention discloses a method for pre...
![benzyl {(1S)-2-[3,5-bis(trifluoromethyl)phenyl]-1-methyl-2-oxoethyl}carbamate](/upload/2023/5\5c2b980e-2cd7-47bc-b6a7-2e35d8f19d26.png)
benzyl {(1S)-2-[3,5-bis(trifluoromethyl)phenyl]-1-methyl-2-oxoethyl}carbamate

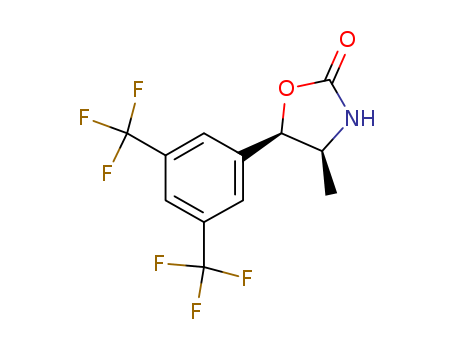
![(4S,5R)-5-[3,5-bis(trifluoromethyl)phenyl]-4-methyl-1,3-oxazolidin-2-one](/upload/2023/5\416b6701-ea82-4d5b-bc70-af0fc74f6875.png)
(4S,5R)-5-[3,5-bis(trifluoromethyl)phenyl]-4-methyl-1,3-oxazolidin-2-one
| Conditions | Yield |
|---|---|
|
benzyl {(1S)-2-[3,5-bis(trifluoromethyl)phenyl]-1-methyl-2-oxoethyl}carbamate; With Dimethylphenylsilane; trifluoroacetic acid; at -6 - 0 ℃; for 16 - 21h;
With potassium hydroxide; In water; at 20 - 30 ℃;
|
99.9% |
|
benzyl {(1S)-2-[3,5-bis(trifluoromethyl)phenyl]-1-methyl-2-oxoethyl}carbamate; With aluminum isopropoxide; In isopropyl alcohol; toluene; at 50 ℃; for 12h; Inert atmosphere;
With potassium hydroxide; In water; isopropyl alcohol; toluene; at 20 ℃; for 3h;
|
91% |
|
benzyl {(1S)-2-[3,5-bis(trifluoromethyl)phenyl]-1-methyl-2-oxoethyl}carbamate; With aluminum isopropoxide; In Isopropyl acetate; toluene; at 50 ℃; for 15.5h;
With potassium hydroxide; In Isopropyl acetate; toluene; at 25 ℃; for 2h;
|
|
|
Multi-step reaction with 2 steps
1: aluminum isopropoxide / toluene; isopropyl alcohol / 15 h / 50 °C
2: potassium hydroxide / isopropyl alcohol / 4 h / 20 °C
With aluminum isopropoxide; potassium hydroxide; In isopropyl alcohol; toluene;
|
|
|
benzyl {(1S)-2-[3,5-bis(trifluoromethyl)phenyl]-1-methyl-2-oxoethyl}carbamate; With aluminum isopropoxide; In isopropyl alcohol; toluene; at 50 ℃; for 15.5h;
With potassium hydroxide; In isopropyl alcohol; toluene; at 20 - 25 ℃; for 2h;
With hydrogenchloride; In water; isopropyl alcohol; toluene; at 25 ℃;
|
|
|
Multi-step reaction with 2 steps
1.1: Dimethylphenylsilane; trifluoroacetic acid / -5 °C
1.2: 0 °C
2.1: water; potassium hydroxide / tetrahydrofuran; methanol / 3 h / 20 °C
With Dimethylphenylsilane; water; trifluoroacetic acid; potassium hydroxide; In tetrahydrofuran; methanol;
|
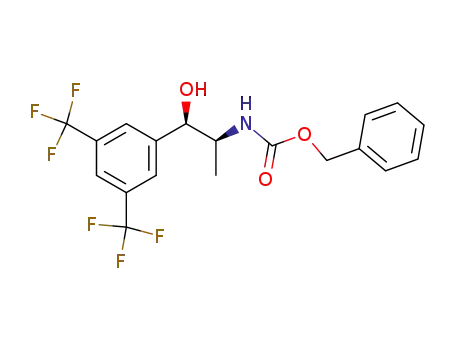
benzyl (1S,2R)-2-(3,5-bis(trifluoromethyl)phenyl)-2-hydroxy-1-methylethylcarbamate

![(4S,5R)-5-[3,5-bis(trifluoromethyl)phenyl]-4-methyl-1,3-oxazolidin-2-one](/upload/2023/5\416b6701-ea82-4d5b-bc70-af0fc74f6875.png)
(4S,5R)-5-[3,5-bis(trifluoromethyl)phenyl]-4-methyl-1,3-oxazolidin-2-one
| Conditions | Yield |
|---|---|
|
With potassium hydroxide; In isopropyl alcohol; at 20 ℃; for 4h;
|
90% |
|
With isopropyl alcohol; potassium hydroxide; In toluene; at 10 - 20 ℃; for 2h;
|
85.2% |
|
With potassium hydroxide; In tetrahydrofuran; water; at 30 ℃; for 3h;
|
99.95 % ee |
|
With potassium hydroxide; In isopropyl alcohol; toluene; at 0 - 20 ℃; for 1h; Temperature;
|
34.9 g |

benzyl (1S,2R)-2-(3,5-bis(trifluoromethyl)phenyl)-2-hydroxy-1-methylethylcarbamate
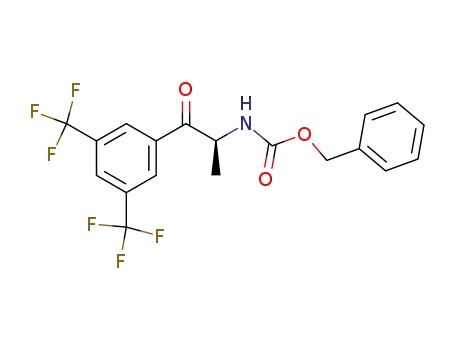
benzyl {(1S)-2-[3,5-bis(trifluoromethyl)phenyl]-1-methyl-2-oxoethyl}carbamate
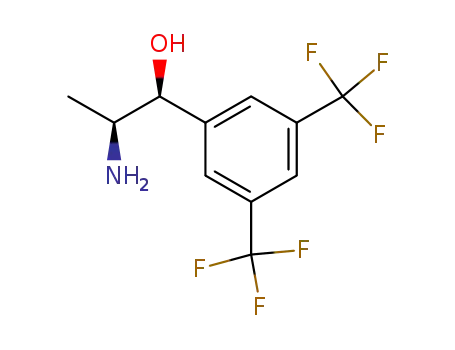
(1S,2S)-2-amino-1-(3,5-bis(trifluoromethyl)phenyl)propane-ol
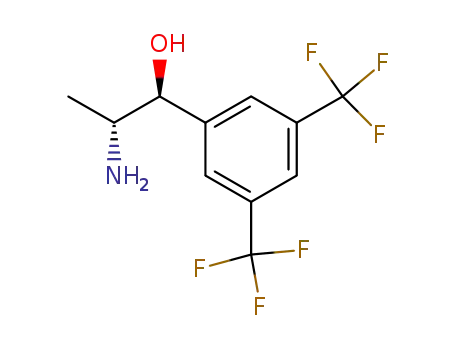
(1S,2R)-2-amino-1-[3,5-bis(trifluoromethyl)phenyl]propan-1-ol
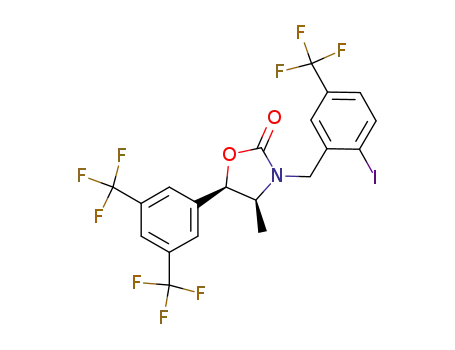
(4S,5R)-5-[3,5-bis(trifluoromethyl)phenyl]-3-[2-iodo-5-(trifluoromethyl)benzyl]-4-methyl-1,3-oxazolidin-2-one
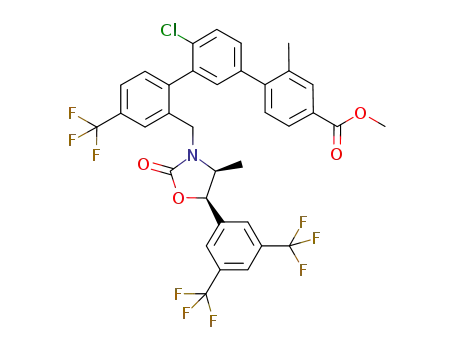
methyl 2''-({(4S,5R)-5-[3,5-bis(trifluoromethyl)phenyl]-4-methyl-2-oxo-1,3-oxazolidin-3-yl}methyl)-4'-chloro-2-methyl-4''-(trifluoromethyl)-1,1':3',1''-terphenyl-4-carboxylate
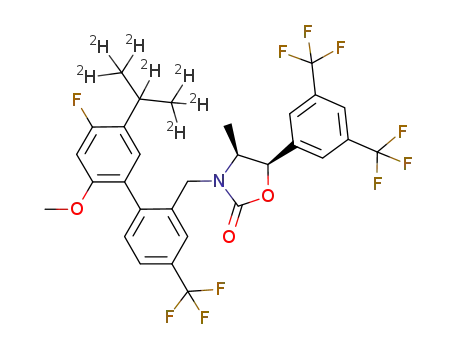
(4S,5R)-5-(3,5-bis(trifluoromethyl)phenyl)-3-((4'-fluoro-5'-(1,1,1,2,3,3,3-d7)-isopropyl-2'-methoxy-4-(trifluoromethyl)biphenyl-2-yl)methyl)-4-methyl-1,3-oxazolidin-2-one
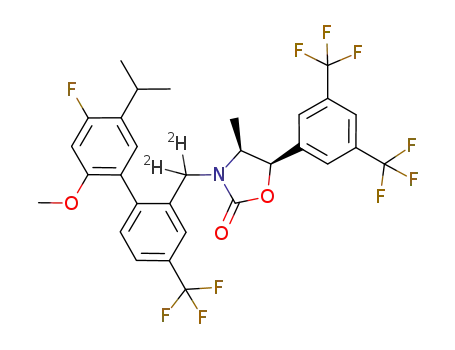
(4S,5R)-5-(3,5-bis(trifluoromethyl)phenyl)-3-((4'-fluoro-5'-isopropyl-2'-methoxy-4-(trifluoromethyl)biphenyl-2-yl)d2-methyl)-4-methyl-1,3-oxazolidin-2-one