Chiral (η6-p-cymene)ruthenium(II) complexes containing monodentate acylthiourea ligands for efficient asymmetric transfer hydrogenation of ketones
Sheeba, Mani Mary,Muthu Tamizh, Manoharan,Farrugia, Louis J.,Endo, Akira,Karvembu, Ramasamy
, p. 540 - 550 (2014)
The new chiral ligands (R)-/(S)-N-((1-ph...
Boron containing chiral Schiff bases: Synthesis and catalytic activity in asymmetric transfer hydrogenation (ATH) of ketones
Pa?a, Salih,Arslan, Nevin,Meri??, Nermin,Kayan, Cezmi,Bingül, Murat,Durap, Feyyaz,Aydemir, Murat
, (2020)
Asymmetric Transfer Hydrogenation (ATH) ...
Production of enantiomerically enriched chiral carbinols using Weissella paramesenteroides as a novel whole cell biocatalyst
Tozlu, Caner,?ahin, Engin,Serencam, Hüseyin,Dertli, Enes
, p. 388 - 398 (2019)
In this study, four bacterial strains we...
Highly Enantioselective Transfer Hydrogenation of Prochiral Ketones Using Ru(II)-Chitosan Catalyst in Aqueous Media
Sz?ll?si, Gy?rgy,Kolcsár, Vanessza Judit
, p. 820 - 830 (2019)
Unprecedentedly high enantioselectivitie...
A new class of well-defined ruthenium catalysts for enantioselective transfer hydrogenation of various ketones
Kayan, Cezmi,Meri?, Nermin,Rafikova, Khadichakhan,Zazybin, Alexey,Gürbüz, Nevin,Karakaplan, Mehmet,Aydemir, Murat
, p. 37 - 47 (2018)
A pair of novel optically pure phosphini...
Asymmetric reduction of prochiral aromatic and hetero aromatic ketones using whole-cell of Lactobacillus senmaizukei biocatalyst
?olak, Nida Sezin,Kalay, Erbay,?ahin, Engin
, p. 2305 - 2315 (2021)
Asymmetric bioreduction of aromatic and ...
Catalysts for the asymmetric transfer hydrogenation of various ketones from [3-[(2S)-2-[(diphenylphosphanyl)oxy]-3-phenoxypropyl]-1-methyl-1H-imidazol-3-ium chloride] and [Ru(η6-arene)(μ-Cl)Cl]2, Ir(η5-C5Me5)(μ-Cl)Cl]2 or [Rh(μ-Cl)(cod)]2
Meri?, Nermin,Arslan, Nevin,Kayan, Cezmi,Rafikova, Khadichakhan,Zazybin, Alexey,Kerimkulova, Aygul,Aydemir, Murat
, p. 108 - 118 (2019)
The combination of [3-[(2S)-2-[(diphenyl...
Application of copper(II)-dipyridylphosphine catalyst in the asymmetric hydrosilylation of simple ketones in air
Zhang, Xi-Chang,Wu, Yan,Yu, Feng,Wu, Fei-Fei,Wu, Jing,Chan, Albert S. C.
, p. 5888 - 5891 (2009)
Copper (II)-dipyridylphosphine catalyst ...
New functional chiral P-based ligands and application in ruthenium-catalyzed enantioselective transfer hydrogenation of ketones
Meri?, Nermin,Kayan, Cezmi,Gürbüz, Nevin,Karakaplan, Mehmet,Binbay, Nil Ertekin,Aydemir, Murat
, p. 1739 - 1749 (2017)
Metal-catalyzed asymmetric transfer hydr...
Bioreduction of prochiral ketones by growing cells of Lasiodiplodia theobromae: Discovery of a versatile biocatalyst for asymmetric synthesis
Barros-Filho, Bartholomeu A.,Nunes, Fatima M.,de Oliveira, Maria da Conceicao F.,Lemos, Telma L.G.,de Mattos, Marcos C.,de Gonzalo, Gonzalo,Gotor-Fernandez, Vicente,Gotor, Vicente
, p. 37 - 40 (2010)
Growing cells of the phytopathogen fungu...
Enantioselective reduction of aryl and hetero aryl methyl ketones using plant cell suspension cultures of Vigna radiata
Santhanam, Srinath,Patil, Swati,Shanmugam, Ramu,Dronamraju V.L, Sarada,Balasundaram, Usha,Baburaj, Baskar
, p. 223 - 229 (2017)
Vigna radiata was investigated as whole ...
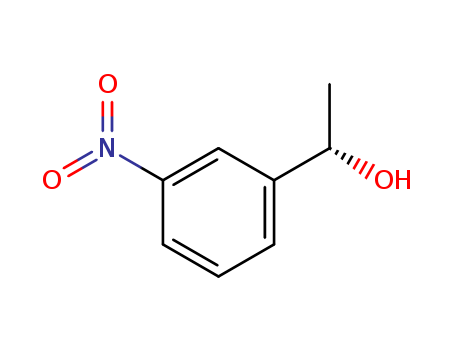
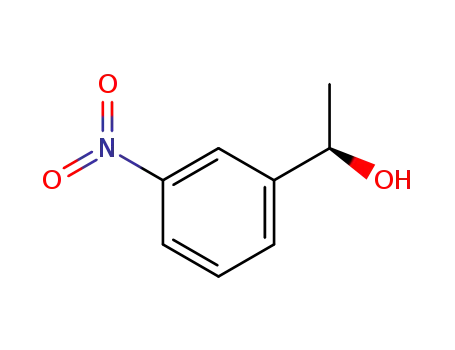
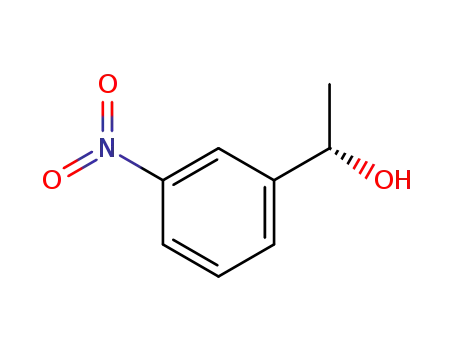
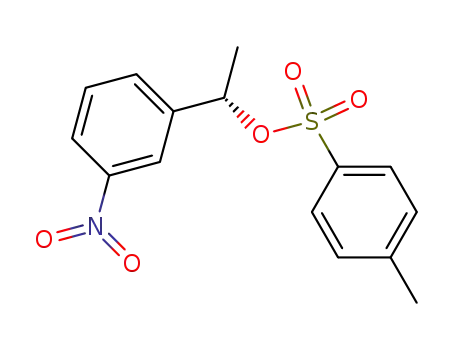
When does an intermediate become a transition state? Degenerate isomerization without competing racemization during solvolysis of (S)-1-(3-nitrophenyl)ethyl tosylate
Tsuji, Yutaka,Richard, John P.
, p. 17139 - 17145 (2006)
(S)-1-(3-Nitrophenyl)ethyl tosylate [(S)...
Candida tropicalis CE017: A new Brazilian enzymatic source for the bioreduction of aromatic prochiral ketones
Vieira, Gizelle A. B.,De Freitas Araujo, Daniel M.,Lemos, Telma L. G.,De Mattos, Marcos Carlos,Da Conceic?a?o F. De Oliveira, Maria,Melo, Va?nia M. M.,De Gonzalo, Gonzalo,Gotor-Ferna?ndez, Vicente,Gotor, Vicente
, p. 1509 - 1516 (2010)
The reactivity and stereoselectivity sho...
Mechanochemical, Water-Assisted Asymmetric Transfer Hydrogenation of Ketones Using Ruthenium Catalyst
Kolcsár, Vanessza Judit,Sz?ll?si, Gy?rgy
, (2022/01/04)
Asymmetric catalytic reactions are among...
Arene-Immobilized Ru(II)/TsDPEN Complexes: Synthesis and Applications to the Asymmetric Transfer Hydrogenation of Ketones
Doherty, Simon,Knight, Julian G.,Alshaikh, Hind,Wilson, James,Waddell, Paul G.,Wills, Corinne,Dixon, Casey M.
supporting information, p. 226 - 235 (2020/12/31)
The Noyori-Ikariya (arene)Ru(II)/TsDPEN ...