- +86 185 8909 5435
- sales@senovaph.com
Your Location:Home >Products >Pharmaceutical intermediates >101403-24-1
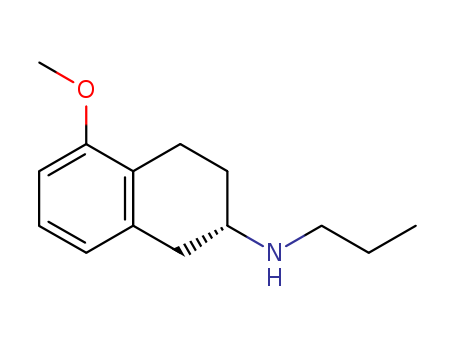

Purity:99%
InChI:InChI=1/C14H21NO/c1-3-9-15-12-7-8-13-11(10-12)5-4-6-14(13)16-2/h4-6,12,15H,3,7-10H2,1-2H3/t12-/m0/s1
Rotigotine is a launched drug for the tr...
The optically pure enantiomers of the po...
2-Aminotetralin and 3-aminochroman deriv...
The invention relates to the technical f...
The invention discloses a preparation me...
A new protocol for the synthesis of chir...
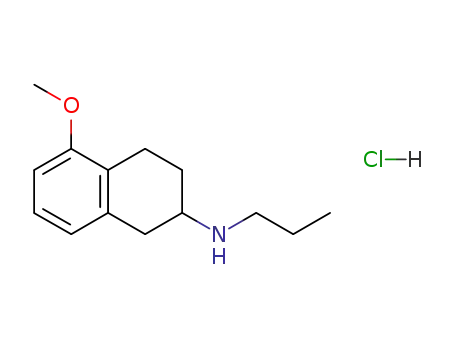
(5-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)propylamine hydrochloride

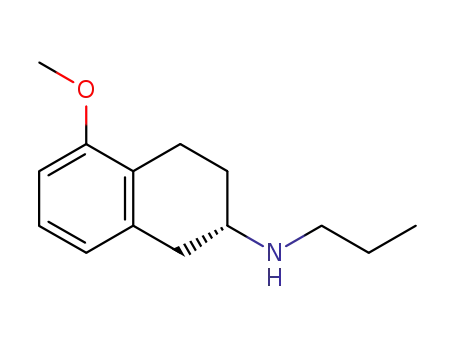
(S)-(5-methoxy-1,2,3,4-tetrahydro-naphthalen-2-yl)-propylamine hydrochloride
| Conditions | Yield |
|---|---|
|
With (S)-(+)-N-(3,5-dinitrobenzoyl)-α-phenylglycine; In water; acetonitrile; at 0 - 5 ℃; for 2h;
|
98% |
|
|
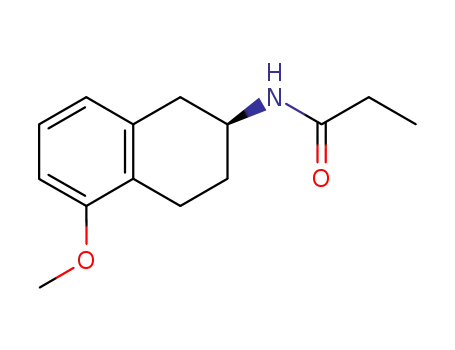
(S)-N-(5-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)propionamide


(S)-(5-methoxy-1,2,3,4-tetrahydro-naphthalen-2-yl)-propylamine hydrochloride
| Conditions | Yield |
|---|---|
|
With lithium aluminium tetrahydride; In tetrahydrofuran; for 42h; Heating;
|
95% |
|
With sodium tetrahydroborate; boron trifluoride diethyl etherate; In tetrahydrofuran; at 0 ℃; for 5h; Reflux;
|
92.5% |

(5-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)propylamine hydrochloride
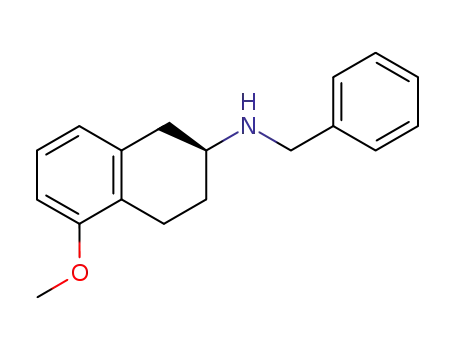
(S)-(-)-2-(N-benzylamino)-5-methoxytetralin
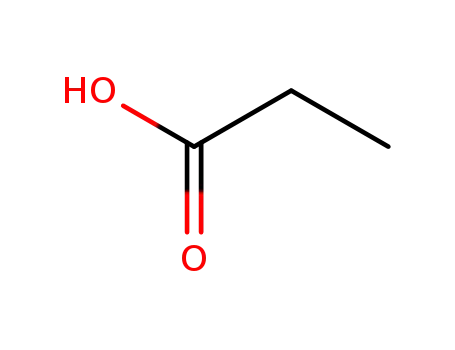
propionic acid
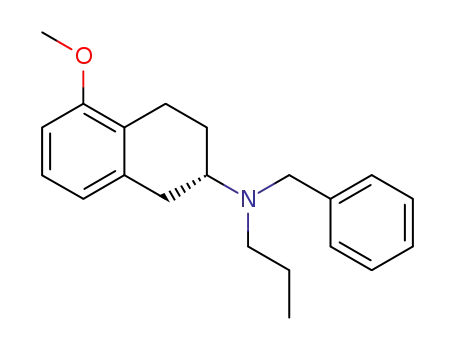
(-)-(2S)-2-(N-benzyl-N-n-propylamino)-5-methoxytetralin
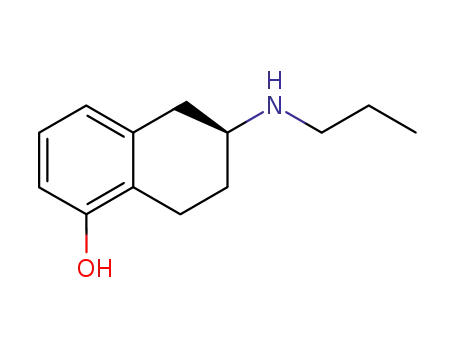
Desthienyl-Rotigotine
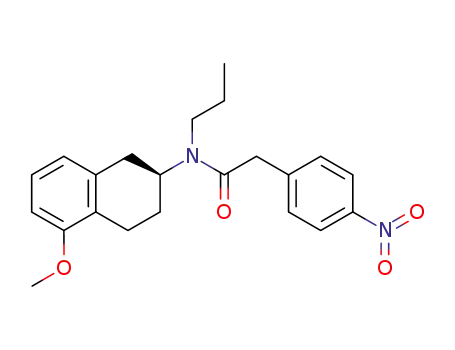
N-((S)-5-Methoxy-1,2,3,4-tetrahydro-naphthalen-2-yl)-2-(4-nitro-phenyl)-N-propyl-acetamide
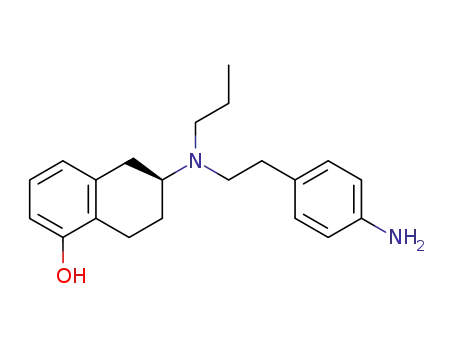
(S)-(-)-2-
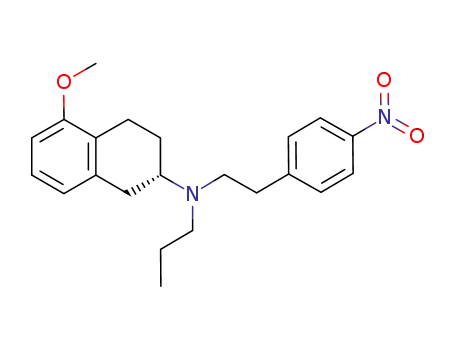
(S)-(-)-2-