- +86 185 8909 5435
- sales@senovaph.com
Your Location:Home >Products >Analytical Chemistry >42177-25-3
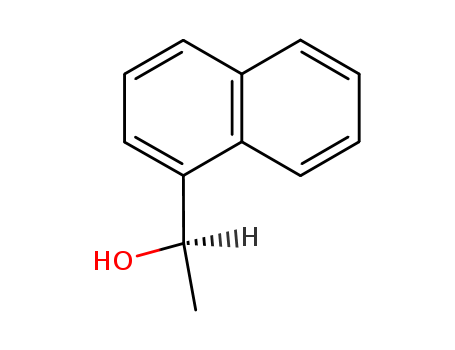

pd_meltingpoint:47-49 °C(lit.)
Purity:99%
|
Chemical Properties |
White solid |
|
Uses |
(R)?-?1-?(Naphthalen-?1-?yl)?ethanol is a coupling reagent used in the synthesis of ferrocene aminophosphoxazoline ligands. |
|
Purification Methods |
Purify the alcohol by recrystallisation from Et2O/pet ether, Et2O, hexane [Balfe et al. J Chem Soc 797 1946, IR, NMR: Theisen & Heathcock J Org Chem 53 2374 1988, see also Fredga et al. Acta Chem Scand 11 1609 1957]. The RS-alcohol [57605-95-5] has m 63-65o, 65-66o from hexane. [Beilstein 6 III 3034, 6 IV 4346.] |
InChI:InChI=1/C12H12O/c1-9(13)11-8-4-6-10-5-2-3-7-12(10)11/h2-9,13H,1H3/t9-/m1/s1
Deracemization of racemic chiral compoun...
Helically chiral poly(quinoxaline-2,3-di...
The invention discloses a tridentate nit...
We report herein an efficient aluminum-c...
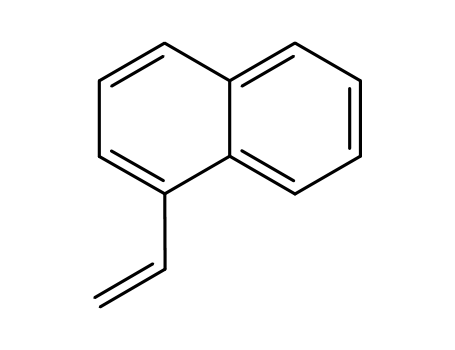
1-vinylnaphthalene

2-naphthaleneethanol

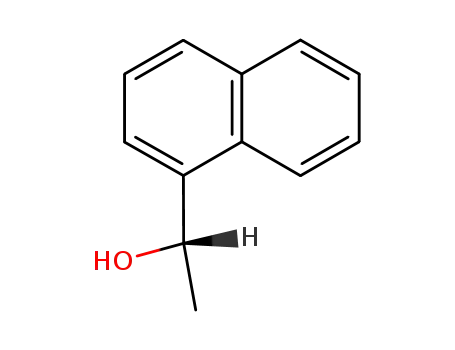
(S)-1-(1-Naphthyl)ethanol

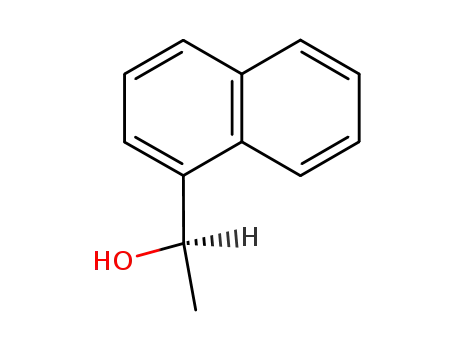
(R)-1-(naphth-1-yl)ethanol
| Conditions | Yield |
|---|---|
|
1-vinylnaphthalene; With benzo[1,3,2]dioxaborole; rhodium complex 2; In toluene; at 20 ℃; for 2h;
With sodium hydroxide; water; dihydrogen peroxide; In toluene; at 20 ℃; for 2h; Title compound not separated from byproducts;
|
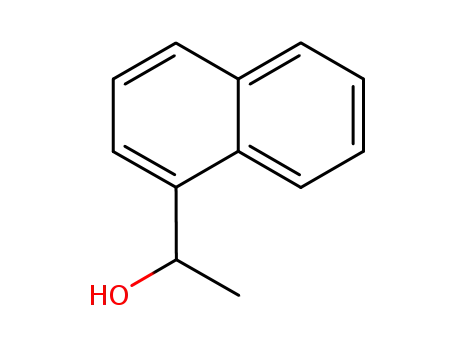
1-(1-naphthyl)ethanol

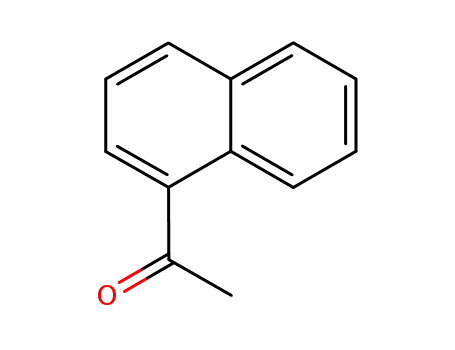
1'-naphthacetophenone


(S)-1-(1-Naphthyl)ethanol


(R)-1-(naphth-1-yl)ethanol
| Conditions | Yield |
|---|---|
|
With (1R)-N-oxyl-1-(N-benzylcarbamoyl)-8-azabicyclo[3.2.1]octane; sodium hydrogencarbonate; sodium bromide; In dichloromethane; water; at 0 ℃; optical yield given as %ee; enantioselective reaction; Electrochemical reaction;
|
62% |
|
With palladium dichloro (η-2,5-norbornadiene); oxygen; (-)-sparteine; In toluene; at 80 ℃; for 192h; Title compound not separated from byproducts;
|
54% |
|
With 3 A molecular sieve; oxygen; (-)-sparteine; palladium dichloro (η-2,5-norbornadiene); In toluene; at 80 ℃; for 192h; under 760 Torr;
|
54% |
|
With Na-Pi buffer; In acetone; for 48h; Yields of byproduct given. Title compound not separated from byproducts; tubers of Solanum tuberosum cv. Saturna, pH 5.9; other biologically active material: tubers of Helianthus tuberosus;
|
10 % Chromat. |
|
With 3 A molecular sieve; oxygen; caesium carbonate; (R)-H8-1,1'-binaphthalenyl-2,2'-diamine-based Pd(II); In toluene; at 80 ℃; for 48h; under 760 Torr;
|
|
|
With oxygen; potassium carbonate; heptakis(6-amino-6-deoxy)-β-cyclodextrin; In tetrahydrofuran; water; at 29.84 ℃; for 48h; enantioselective reaction; Resolution of racemate;
|

1'-naphthacetophenone
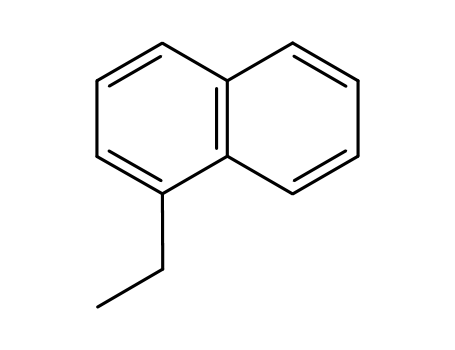
1-ethylnapthelene

1-(1-naphthyl)ethanol
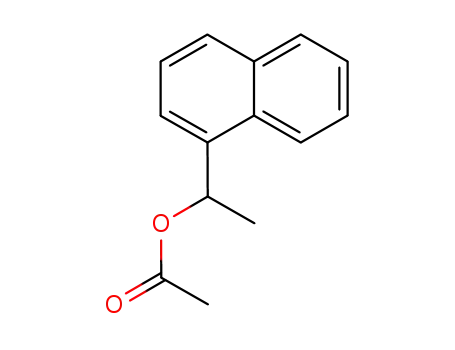
acetic acid 1-(1-naphthyl)ethyl ester
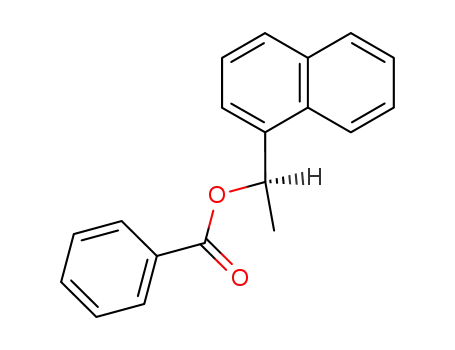
(R)-1-(naphthalen-1-yl)ethyl benzoate

1-(1-naphthyl)ethanol