- +86 185 8909 5435
- sales@senovaph.com
Your Location:Home >Products >Organic Chemistry >1032903-50-6
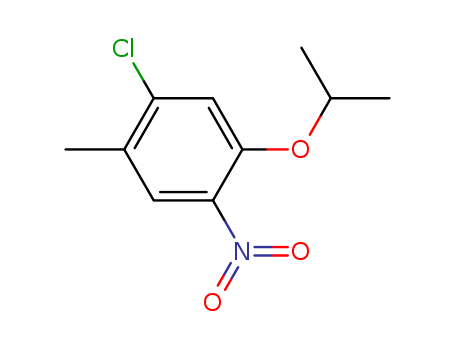

Purity:99%
InChI:InChI=1S/C10H12ClNO3/c1-6(2)15-10-5-8(11)7(3)4-9(10)12(13)14/h4-6H,1-3H3
The invention relates to the field of me...
Disclosed are a condensed-ring pyrimidyl...
The present invention relates to 2-isopr...
The invention relates to a preparation m...
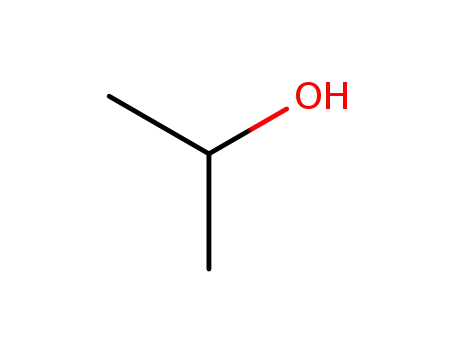
isopropyl alcohol

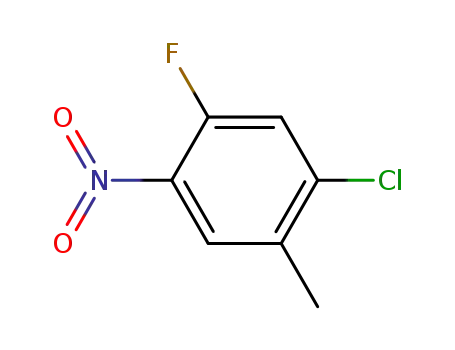
1-chloro-5-fluoro-2-methyl-4-nitrobenzene

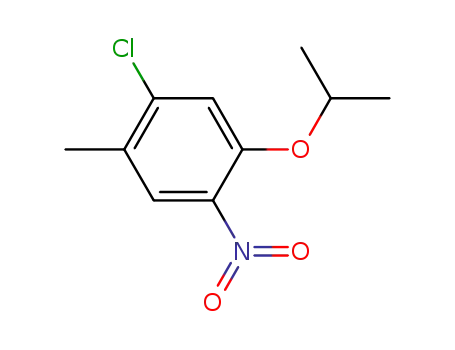
1-chloro-2-methyl-4-nitro-5-(propan-2-yloxy)benzene
| Conditions | Yield |
|---|---|
|
With caesium carbonate; at 60 ℃;
|
95% |
|
With caesium carbonate; at 60 ℃; for 16h;
|
95% |
|
With potassium hydroxide; at 20 ℃;
|
86.3% |
|
With caesium carbonate; for 10h; Reflux;
|
83% |
|
With caesium carbonate; at 60 ℃;
|
82% |
|
With caesium carbonate; at 60 ℃;
|
82% |
|
With caesium carbonate; at 60 ℃; for 24h;
|
74.4% |
|
With caesium carbonate; at 60 ℃;
|
|
|
With potassium carbonate; for 40h; Reflux;
|
|
|
With potassium carbonate; for 40h; Reflux;
|
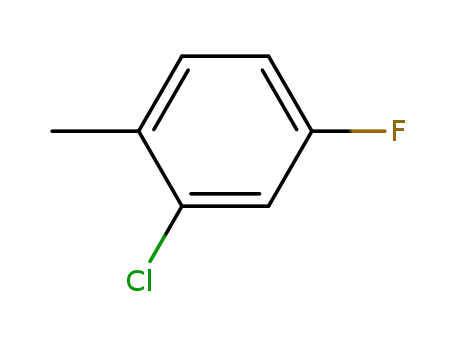
2-chloro-4-fluorotoluene


1-chloro-2-methyl-4-nitro-5-(propan-2-yloxy)benzene
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: sulfuric acid; potassium nitrate / 0 - 20 °C
2: caesium carbonate / 60 °C
With sulfuric acid; caesium carbonate; potassium nitrate;
|
|
|
Multi-step reaction with 2 steps
1: sulfuric acid; potassium nitrate / 22 h / 0 - 20 °C
2: caesium carbonate / 24 h / 60 °C
With sulfuric acid; caesium carbonate; potassium nitrate;
|
|
|
Multi-step reaction with 2 steps
1: sulfuric acid; nitric acid / Cooling with ice
2: potassium carbonate / 40 h / Reflux
With sulfuric acid; nitric acid; potassium carbonate;
|
|
|
Multi-step reaction with 2 steps
1: sulfuric acid; nitric acid / Cooling with ice
2: potassium carbonate / 40 h / Reflux
With sulfuric acid; nitric acid; potassium carbonate;
|

isopropyl alcohol

1-chloro-5-fluoro-2-methyl-4-nitrobenzene

2-chloro-4-fluorotoluene
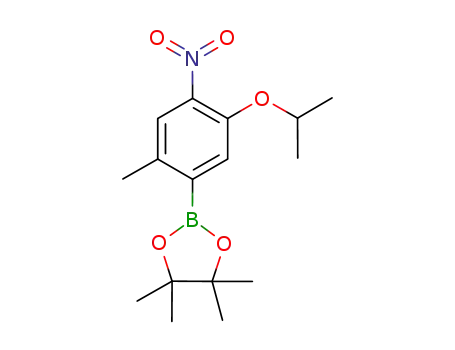
2-(5-isopropoxy-2-methyl-4-nitrophenyl)-4,4,5,5-tetramethyl-1,3,2-dioxide cyclopentaborane
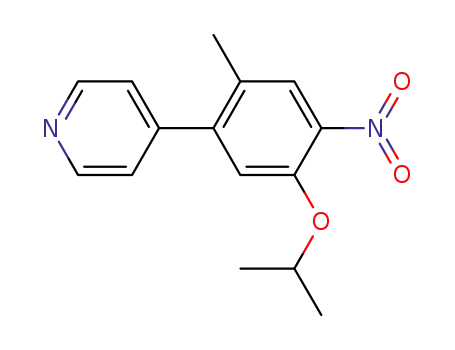
4-[2-methyl-4-nitro-5-(propan-2-yloxy)phenyl]pyridine
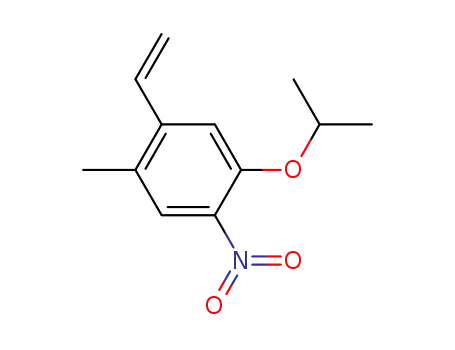
1-methyl-5-nitro-4-propoxy-2-vinyl-benzene
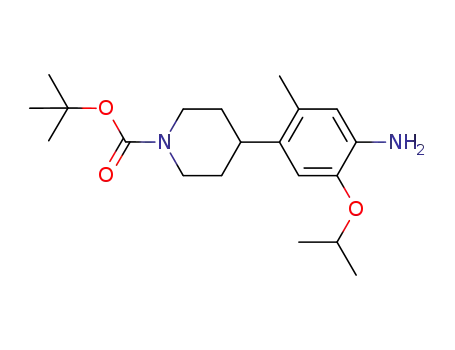
4-(4-amino-5-isopropoxy-2-methylphenyl)-piperidine-1-carboxylic acid tert-butyl ester