- +86 185 8909 5435
- sales@senovaph.com
Your Location:Home >Products >Pharmaceutical intermediates >877397-65-4

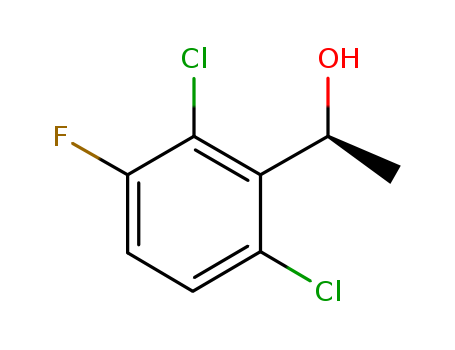
Purity:99%
|
Article |
Source |
|
Uses |
(S)-1-(2,6-Dichloro-3-fluorophenyl)ethanol is an intermediate in the synthetic preparation of Crizotinib (C785000), a potent and selective dual inhibitor of mesenchymal-epithelial transition factor ( c-MET) kinase and anaplastic lymphoma kinase (ALK). Crizotinib is a potential antitumor agent. |
InChI:InChI=1/C8H7Cl2FO/c1-4(12)7-5(9)2-3-6(11)8(7)10/h2-4,12H,1H3/t4-/m0/s1
(S)-1-(2, 6-dichloro-3-fluorophenyl) eth...
Enzymatic reactions through mononuclear ...
The invention relates to a synthesis met...
The invention relates to a preparation m...
A series of Mn(I) catalysts containing i...
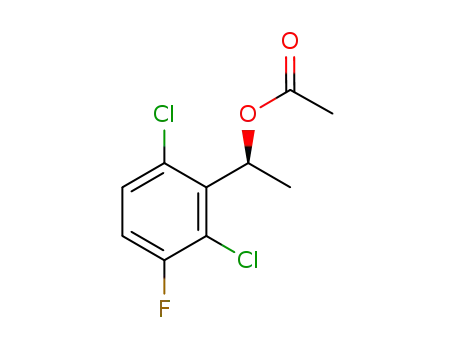
(1S)-1-(2,6-dichloro-3-fluorophenyl)ethyl acetate

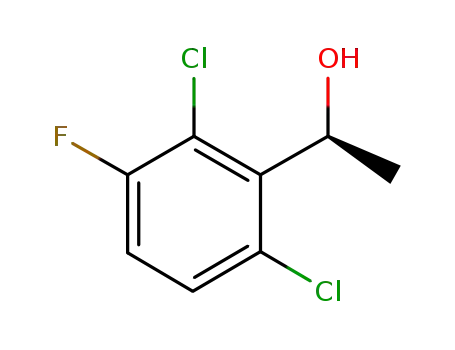
(S)‐1‐(2,6-dichloro-3-fluorophenyl)ethanol
| Conditions | Yield |
|---|---|
|
(1S)-1-(2,6-dichloro-3-fluorophenyl)ethyl acetate; With sodium methylate; In methanol; at 0 - 20 ℃; for 4h;
With sodium acetate; acetic acid; In water; pH=7;
|
94.9% |
|
With sodium methylate; In methanol; at 0 - 20 ℃; for 4h;
|
94.4% |
|
With methanol; sodium methylate; at 0 - 20 ℃; for 4h;
|
94.9% |
|
(1S)-1-(2,6-dichloro-3-fluorophenyl)ethyl acetate; With sodium methylate; In methanol; at 0 - 20 ℃; for 4h;
With water; In methanol; pH=7; sodium acetate-acetic acid buffer;
|
94.9% |
|
With lithium hydrochloride monohydrate; In methanol; at 0 - 5 ℃; for 0.75h;
|
94% |
|
With sodium methylate; In methanol; at 0 - 20 ℃; for 4h; Inert atmosphere;
|
94.9% |
|
With sodium methylate; In methanol; at 0 - 20 ℃; for 4h; Inert atmosphere;
|
94.9% |
|
With sodium methylate; In methanol; at 0 - 20 ℃; for 4h; Inert atmosphere;
|
94.9% |
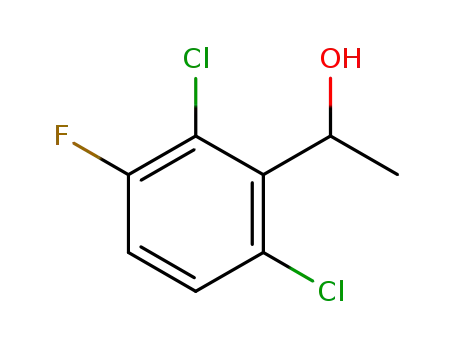
1-(2,6-dichloro-3-fluorophenyl)ethanol


(S)‐1‐(2,6-dichloro-3-fluorophenyl)ethanol
| Conditions | Yield |
|---|---|
|
In Petroleum ether; at 40 ℃; for 5.25h; Inert atmosphere;
|
66.7% |
|
With dmap; 1-(tert-butoxycarbonyl)-L-proline; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; In 1,2-dichloro-ethane; at 0 - 20 ℃;
|
65.6% |
|
With dmap; 1-(tert-butoxycarbonyl)-L-proline; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; In 1,2-dichloro-ethane; at 0 - 20 ℃;
|
65.6% |
|
With dmap; 1-(tert-butoxycarbonyl)-L-proline; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; In 1,2-dichloro-ethane; at -5 ℃;
|
58% |
|
Multi-step reaction with 3 steps
1: pyridine / dichloromethane / 12 h / 20 °C
2: sodium hydroxide / aq. phosphate buffer / 20 h / 20 °C / pH 7.0 / Enzymatic reaction
3: sodium methylate / methanol / 4 h / 0 - 20 °C / Inert atmosphere
With pyridine; sodium methylate; sodium hydroxide; In methanol; aq. phosphate buffer; dichloromethane;
|
|
|
Multi-step reaction with 5 steps
1: pyridine / dichloromethane / 12 h / 20 °C
2: sodium hydroxide / aq. phosphate buffer / 20 h / 20 °C / pH 7.0 / Enzymatic reaction
3: pyridine / 3 h / 20 °C / Inert atmosphere
4: N,N-dimethyl-formamide / 12 h / 100 °C / Inert atmosphere
5: sodium methylate / methanol / 4 h / 0 - 20 °C / Inert atmosphere
With pyridine; sodium methylate; sodium hydroxide; In methanol; aq. phosphate buffer; dichloromethane; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 3 steps
1.1: pyridine / dichloromethane / 12 h / 20 °C
2.1: sodium hydroxide / aq. phosphate buffer / 20 h / 20 °C / pH 7
2.2: 3 h / 20 °C / Inert atmosphere
2.3: 12 h / 100 °C / Inert atmosphere
3.1: sodium methylate / methanol / 4 h / 0 - 20 °C / Inert atmosphere
With pyridine; sodium methylate; sodium hydroxide; In methanol; aq. phosphate buffer; dichloromethane;
|
|
|
Multi-step reaction with 3 steps
1.1: pyridine / dichloromethane / 12 h / 20 °C
2.1: sodium hydroxide / aq. phosphate buffer / 20 h / 20 °C / pH 7
2.2: 3 h / 20 °C / Inert atmosphere
3.1: sodium methylate / methanol / 4 h / 0 - 20 °C / Inert atmosphere
With pyridine; sodium methylate; sodium hydroxide; In methanol; aq. phosphate buffer; dichloromethane;
|
|
|
Multi-step reaction with 3 steps
1: pyridine / dichloromethane / 12 h / 20 °C
2: sodium hydroxide / aq. phosphate buffer / 20 h / 20 °C / pH 7
3: sodium methylate / methanol / 4 h / 0 - 20 °C / Inert atmosphere
With pyridine; sodium methylate; sodium hydroxide; In methanol; aq. phosphate buffer; dichloromethane;
|
|
|
1-(2,6-dichloro-3-fluorophenyl)ethanol; With 1-(tert-butoxycarbonyl)-L-proline; toluene-4-sulfonic acid; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; In dichloromethane; at 0 - 20 ℃; for 2h;
With sodium hydroxide; In methanol; water; at 25 ℃; for 1.5h; Further stages;
|
140 g |
|
Multi-step reaction with 3 steps
1.1: pyridine / dichloromethane / 12 h / 20 °C
2.1: sodium hydroxide / aq. acetate buffer / 20 h / 20 °C / pH 7
2.2: 3 h / 20 °C / Inert atmosphere
2.3: 12 h / 100 °C / Inert atmosphere
3.1: sodium methylate / methanol / 4 h / 0 - 20 °C / Inert atmosphere
With pyridine; sodium methylate; sodium hydroxide; In methanol; dichloromethane;
|
|
|
Multi-step reaction with 3 steps
1.1: pyridine / dichloromethane / 12 h / 20 °C
2.1: sodium hydroxide / aq. acetate buffer / 20 h / 20 °C / pH 7
2.2: 3 h / 20 °C / Inert atmosphere
3.1: sodium methylate / methanol / 4 h / 0 - 20 °C / Inert atmosphere
With pyridine; sodium methylate; sodium hydroxide; In methanol; dichloromethane;
|
|
|
Multi-step reaction with 3 steps
1: pyridine / dichloromethane / 12 h / 20 °C
2: sodium hydroxide / aq. phosphate buffer / 20 h / 20 °C / pH 7
3: sodium methylate / methanol / 4 h / 0 - 20 °C / Inert atmosphere
With pyridine; sodium methylate; sodium hydroxide; In methanol; aq. phosphate buffer; dichloromethane;
|

(1S)-1-(2,6-dichloro-3-fluorophenyl)ethyl acetate

1-(2,6-dichloro-3-fluorophenyl)ethanol
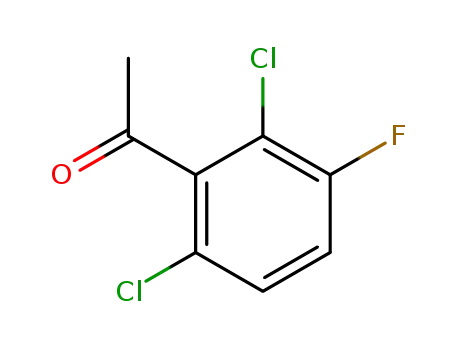
2',6'-dichloro-3'-fluoroacetophenone
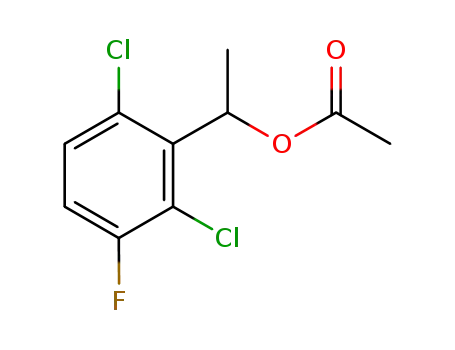
1-(2,6-dichloro-3-fluorophenyl)ethyl acetate
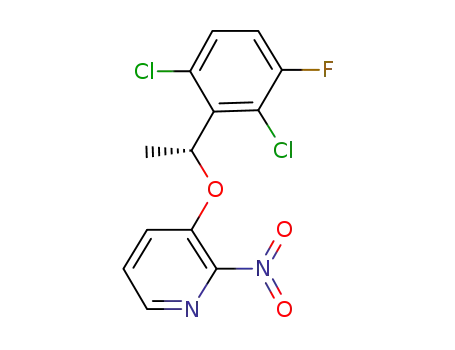
(R)-3-(1-(2,6-dichloro-3-fluorophenyl)ethyoxyl)-2-nitropyridine
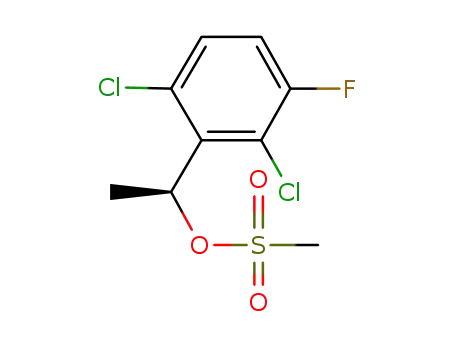
(1S)-1-(2,6-dichloro-3-fluorophenyl)ethyl methanesulfonate
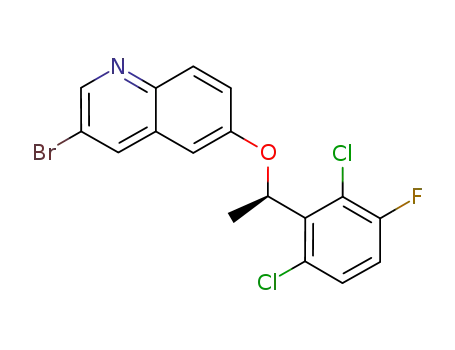
C17H11BrCl2FNO
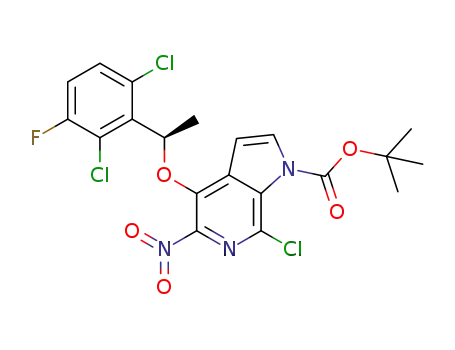
C20H17Cl3FN3O5