- +86 185 8909 5435
- sales@senovaph.com
Your Location:Home >Products >Biochemical Engineering >1268524-70-4

Purity:99%
Development of proteolysis targeting chi...
As one of the most aggressive and lethal...
The present invention relates to a class...
The invention relates to a process for t...
C33H32ClN2O6PS

acetic acid hydrazide

(-)-JQ1

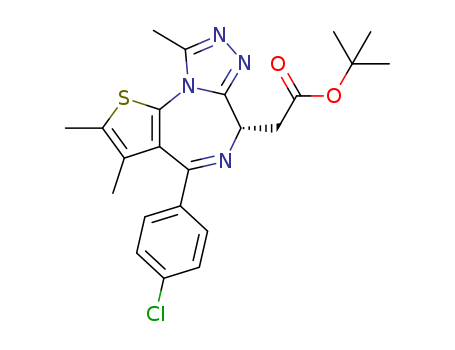
(S)-tert-butyl 2-(4-(4-chlorophenyl)-2,3,9-trimethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetate
| Conditions | Yield |
|---|---|
|
In tetrahydrofuran; butan-1-ol; at 20 - 90 ℃; for 2h; Overall yield = 3.04 g;
|
82 % ee |
Fmoc-(tBu)Asp-OH

(-)-JQ1

(S)-tert-butyl 2-(4-(4-chlorophenyl)-2,3,9-trimethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetate
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 4 steps
1.1: N-ethyl-N,N-diisopropylamine; benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate / N,N-dimethyl-formamide / 23 °C
2.1: piperidine / N,N-dimethyl-formamide / 23 °C
2.2: 90 °C
3.1: potassium tert-butylate / tetrahydrofuran / 0.5 h / -78 - -10 °C
3.2: 0.75 h / -78 - -10 °C
4.1: tetrahydrofuran; butan-1-ol / 2 h / 20 - 90 °C
With piperidine; potassium tert-butylate; benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine; In tetrahydrofuran; N,N-dimethyl-formamide; butan-1-ol;
|
|
|
Multi-step reaction with 5 steps
1.1: N-ethyl-N,N-diisopropylamine; benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate / N,N-dimethyl-formamide / 23 °C
2.1: piperidine / N,N-dimethyl-formamide / 23 °C
3.1: acetic acid / ethanol / 80 °C
4.1: potassium tert-butylate / tetrahydrofuran / 0.5 h / -78 - -10 °C
4.2: 0.75 h / -78 - -10 °C
5.1: tetrahydrofuran; butan-1-ol / 2 h / 20 - 90 °C
With piperidine; potassium tert-butylate; benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; acetic acid; N-ethyl-N,N-diisopropylamine; In tetrahydrofuran; ethanol; N,N-dimethyl-formamide; butan-1-ol;
|
Isopropenyl acetate
ethanol
orthoformic acid triethyl ester
acetone
8-{2-[(S)-4-(4-chlorophenyl)-2,3,9-trimethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl]acetyl}-8-azabicyclo[3.2.1]octan-3-one
2-[(S)-4-(4-chlorophenyl)-2,3,9-trimethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl]-1-(2-oxa-6-azaspiro[3.3]hept-6-yl)ethan-1-one
2-[(S)-4-(4-chlorophenyl)-2,3,9-trimethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl]-1-(8-oxa-3-azabicyclo[3.2.1]oct-3-yl)ethan-1-one
2-[(S)-4-(4-chlorophenyl)-2,3,9-trimethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl]-1-(2-oxa-6-azaspiro[3.4]oct-6-yl)ethan-1-one