- +86 185 8909 5435
- sales@senovaph.com
Your Location:Home >Products >Organic Chemistry >905579-51-3

pd_meltingpoint:161-163 °C
Appearance:White Solid
Purity:99%
|
Description |
MLN4924 (905579-51-3) is a potent and selective NEDD8-activating enzyme (NAE) inhibitor.1?It disrupts cullin-RING ligase-mediated protein turnover leading to apoptosis in human tumor cells. Suppresses the growth of human tumor xenografts in mice.2?Upregulates PD-L1 expression and enhances the efficacy of immune checkpoint blockade in glioblastoma.3?Modulates tumor microenvironment.4?Cell permeable. |
|
Chemical Properties |
White Solid |
|
Uses |
A potent and selective inhibitor of NAE. |
|
Enzyme inhibitor |
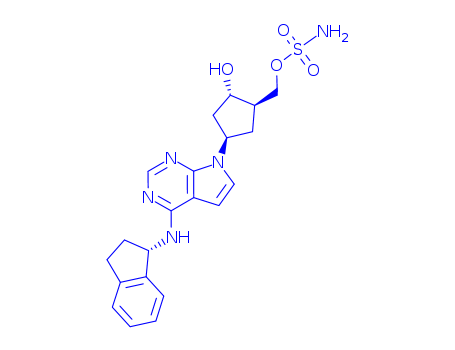
This first-in-class small molecule inhibitor (FW = 443.52 g/mol; CAS 905579-51-3; Solubility = 10 mg/mL DMSO), also named MLN4924 and [(1S,2S,4R)-4-[4-[[(1S)-2,3-dihydro-1H-inden-1-yl]amino]-7H-pyrrolo[2,3- d]pyrimidin-7-yl]-2-hydroxycyclopentyl]sulfamate methyl ester, targets Nedd8 activating enzyme, or NAE (IC50 = 4.7 nM), with much weaker action against UAE (IC50 = 1.5 μM), SAE (IC50 = 8.2 μM), and UBA6 (IC50 = 1.8 μM). In most cancer cells, pevonedistat treatment results in the induction of DNA re-replication, resulting in DNA damage and cell death. MLN4924 also exhibits an alternative mechanism of action. Treatment of activated B cell-like (ABC) diffuse large B-cell lymphoma (DLBCL) cells with pevonedistat resulted in rapid accumulation of pIkBa, decrease in nuclear p65 content, reduction of transcriptional activity of NF-kB (or nuclear factor k-light-chain-enhancer of activated B cells), and G1 arrest, ultimately resulting in apoptosis induction, events consistent with potent NF-kB pathway inhibition. Treatment of germinal-center B cell-like (GCB) DLBCL cells resulted in an increase in cellular Cdt-1 and accumulation of cells in S-phase, consistent with cells undergoing DNA rereplication. Pevonedistat also inhibits Vpx/Vpr-induced SAMHD1 degradation by inhibiting the neddylation of E3 ubiquitin-ligase and blocking SIVmac replication in myeloid cells, therebyindicating the potential efficacy of inhibiting neddylation as an antiretroviral strategy |
|
References |
1) Soucy, et al. (2009), An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature 458 732 2) Milhollen, et al. (2010) MLN4924, a NEDD8-activating enzyme inhibitor, is active in diffuse large B-cell lymphoma models: rationale for treatment of NF-(kappa)B-dependent lymphoma. Blood, 116 1515 3) Zhou et al. (2019) Neddylation inhibition upregulates PD-L1 expression and enhances the efficacy of immune checkpoint blockade in glioblastoma; Int. J. Cancer, 145 763 4) Zhou et al. (2019) Neddylation: a novel modulator of the tumor microenvironment; Mol. Cancer 18 77 |
Sulfamates and sulfamides are prevalent ...
We report a new catalytic method for alc...
A practical synthesis of a novel NEDD8-a...
The present invention relates to a metho...
![tert-butyl {[((1S,2S,4R)-4-{4-[(1R)-2,3-dihydro-1H-inden-1-ylamino]-7H-pyrrolo[2,3-d]pyrimidin-7-yl}-2-hydroxycyclopentyl)methoxy]sulfonyl}carbamate](/upload/2023/6\5ab97bd1-390a-44d8-8ef8-7c4052da46f5.png)
tert-butyl {[((1S,2S,4R)-4-{4-[(1R)-2,3-dihydro-1H-inden-1-ylamino]-7H-pyrrolo[2,3-d]pyrimidin-7-yl}-2-hydroxycyclopentyl)methoxy]sulfonyl}carbamate

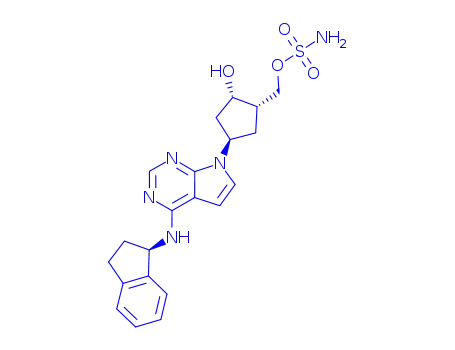
MLN4924
| Conditions | Yield |
|---|---|
|
tert-butyl {[((1S,2S,4R)-4-{4-[(1R)-2,3-dihydro-1H-inden-1-ylamino]-7H-pyrrolo[2,3-d]pyrimidin-7-yl}-2-hydroxycyclopentyl)methoxy]sulfonyl}carbamate; With trifluoroacetic acid; In dichloromethane; at 20 ℃; for 0.25h;
With sodium hydrogencarbonate; In methanol; for 0.166667h;
|
58% |
![(1S,2S,4R)-2-{[(Aminosulfonyl)oxy]methyl}-4-{4-[(1S)-2,3-dihydro-1H-inden-1-ylamino]-7H-pyrrolo[2,3-d]pyrimidin-7-yl}cyclopentyl acetate](/upload/2023/6\663be6b8-5498-43c9-b90c-ad2b6a76ae68.png)
(1S,2S,4R)-2-{[(Aminosulfonyl)oxy]methyl}-4-{4-[(1S)-2,3-dihydro-1H-inden-1-ylamino]-7H-pyrrolo[2,3-d]pyrimidin-7-yl}cyclopentyl acetate

![((1S,2S,4R)-4-(4-(((S)-2,3-dihydro-1H-inden-1-yl)amino)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)-2-hydroxycyclopentyl)-methyl sulfamate](/upload/2023/6\492bd719-c66f-43ab-9f3a-058e563b3c4d.png)
((1S,2S,4R)-4-(4-(((S)-2,3-dihydro-1H-inden-1-yl)amino)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)-2-hydroxycyclopentyl)-methyl sulfamate
| Conditions | Yield |
|---|---|
|
With methanol; ammonia; at 20 ℃; for 120h;
|
90% |
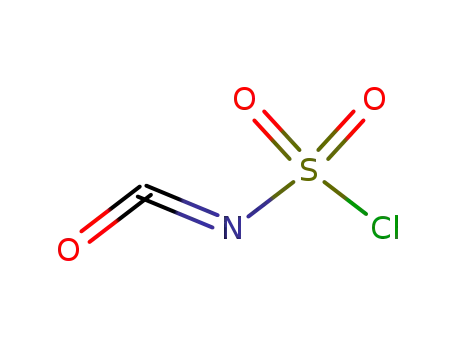
isocyanate de chlorosulfonyle
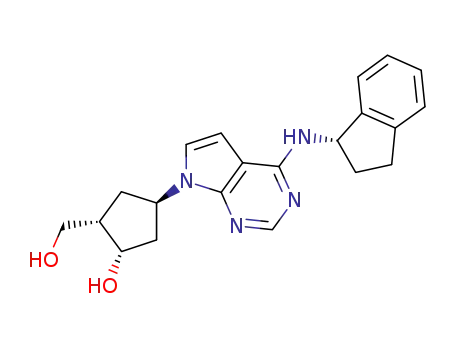
(1S,2S,4R)-4-{4-[(1S)-2,3-dihydro-1H-inden-1-ylamino]-7H-pyrrolo[2,3-d]pyrimidin-7-yl}-2-(hydroxymethyl)-cyclopentanol
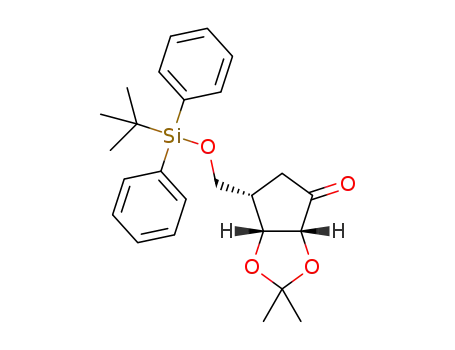
6-(tert-butyldiphenylsilanyloxymethyl)-2,2-dimethyltetrahydrocyclopenta[1,3]dioxol-4-one
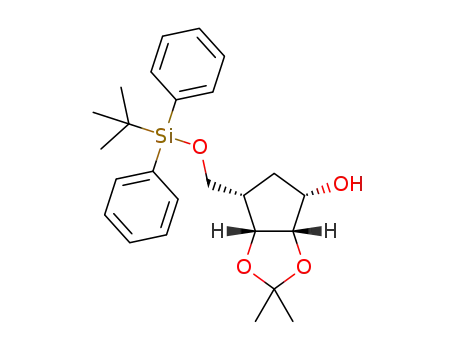
6-(tert-butyldiphenylsilanyloxymethyl)-2,2-dimethyltetrahydrocyclopenta[1,3]dioxol-4-ol