- +86 185 8909 5435
- sales@senovaph.com
Your Location:Home >Products >Biochemical Engineering >118-00-3
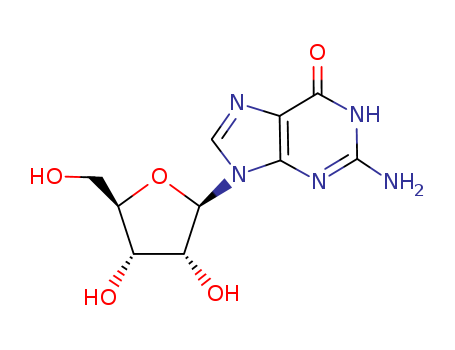

pd_meltingpoint:250 °C (dec.)(lit.)
Appearance:crystalline
Purity:99%
|
Description |
Guanosine is a purine nucleoside, in which the guanine attached to the C1 carbon of a ribose (ribofuranose) ring via a β-N9-glycosidic bond. Its phosphorylated derivatives include GMP (guanosine monophosphate), cGMP (cyclic guanosine monophosphate), GDP (guanosine diphosphate), and GTP (guanosine triphosphate). These guanosine derivatives are very important in various biochemical processes, such as synthesis of nucleic acids and proteins, photosynthesis, muscle contraction, and intracellular signal transduction. Guanosine is thought to have neuroprotective properties. It can reduce neuroinflammation, oxidative stress, and excitotoxicity, as well as exerting trophic effects in neuronal and glial cells.?It is shown to be protective in central nervous system diseases including ischemic stroke, Alzheimer’s disease, Parkinson’s disease, spinal cord injury, nociception, and depression. Guanosine is found to be associated with purine nucleoside phosphorylase (PNP) deficiency, which is an inborn error of metabolism. |
|
Reference |
L. E. B. Bettio, J. Gil-Mohapel, A. L. S. Rodrigues, Guanosine and its role in neurophathologies, Purinergic Signal, 2016, vol. 12, pp. 411-426 |
|
Chemical Properties |
White, crystalline powder; odorless; mild saline taste. Very slightly soluble in cold water; soluble in boiling water, dilute mineral acids, hot acetic acid, and dilute bases; insoluble in alcohol, ether, chloroform, and benzene. |
|
Uses |
A constituent of nucleic acids. |
|
Definition |
A NUCLEOSIDE present in DNA and RNA and consisting of guanine linked to D-ribose via a β-glycosidic bond. |
|
General Description |
Guanosine is an aromatic organic molecule and a purine nucleoside. It is present in the cerebrospinal fluid, intestinal cells, blood-brain barrier and in brain microvessels. |
|
Biochem/physiol Actions |
Guanosine nucleoside elicits cellular effect as the guanine-based purinergic system. It modulates glutamate uptake by glutamate transporters. It may have neuroprotective functionality in central nervous system disorders. Guanosine promotes neurite arborization, outgrowth, proliferation and differentiation. Administration of guanosine replenished GTP and elicits protective function in renal ischemic injury. |
|
Purification Methods |
It crystallises from water as a dihydrate. Dry it at 110o.[Beilstein 26/18 V 81.] |
InChI:InChI=1/C10H13N5O5/c11-10-13-7-4(8(19)14-10)12-2-15(7)9-6(18)5(17)3(1-16)20-9/h2-3,5-6,9,16-18H,1H2,(H3,11,13,14,19)/t3-,5+,6-,9+/m1/s1
A straightforward methodology for deacet...
Treatment of 5-amino-1-β-D-ribofuranosyl...
Primary amines can be converted in high ...
The 5′-phosphorylated oligonucleotides (...
The suitability of the 4-methoxytetrahyd...
-
Chemical radiolytic methods were used to...
-
Hydrolytic reactions of guanosyl-(3′,3′)...
Adenosine kinase is an enzyme catalyzing...
-
Glutathione (GSH) plays an important rol...
1,1,1,3,3,3-Hexafluoro-2-propanol is int...
A 3′-deoxy-3′-C-methylenephosphonate mod...
A short and efficient synthesis of 2'-O-...
A monitoring method of rapid hydrotherma...
Abstract An efficient approach for the d...
A disulfide made by oxidation of 8-thiog...
Watson-Crick base pairing in dimethyl su...
The use of nucleoside phosphorylases (NP...
Vorbrueggen coupling of trimethylsilylat...
Lanthanide ions can mediate both phospho...
-
Thione-containing nucleobases have attra...
In thiourea assisted dissociation of 2+ ...
-
A new modified nucleoside was formed by ...
The development of modular and efficient...
Phosphorolysis catalyzed by Cellulomonas...
The design and implementation of 2′-hydr...
-
Nucleoside analogs represent a class of ...
The present invention provides a method ...
![Acetic acid (2R,3R,4R,5R)-4-acetoxy-5-acetoxymethyl-2-[2-acetylamino-6-(2,4,6-triisopropyl-benzenesulfonyloxy)-purin-9-yl]-tetrahydro-furan-3-yl ester](/upload/2023/6\f13e62f7-0920-4a13-b34e-062265c6186a.png)
Acetic acid (2R,3R,4R,5R)-4-acetoxy-5-acetoxymethyl-2-[2-acetylamino-6-(2,4,6-triisopropyl-benzenesulfonyloxy)-purin-9-yl]-tetrahydro-furan-3-yl ester

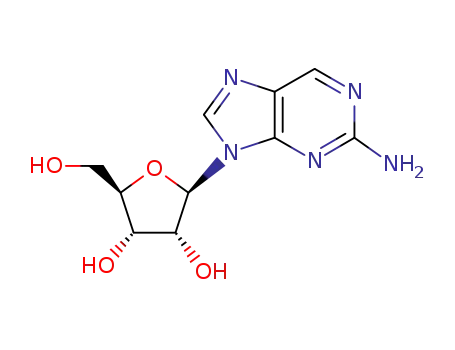
2?amino?9?(β?D?ribofuranosyl)purine

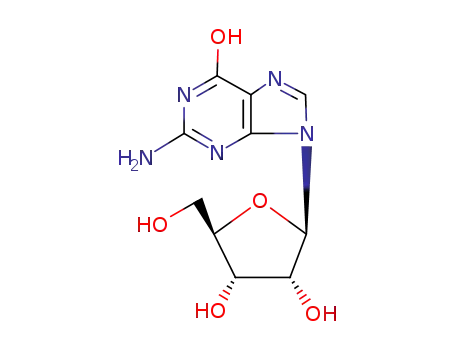
guanosine
| Conditions | Yield |
|---|---|
|
With palladium diacetate; triethylammonium formate; methylamine; Yield given. Multistep reaction. Yields of byproduct given; 1.) dioxane, 90 deg C;
|
highly polymerized dsDNA from salmon testes

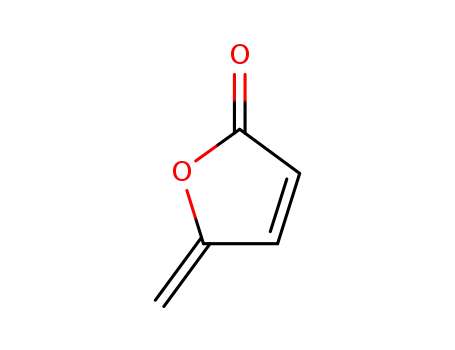
protoanemonin

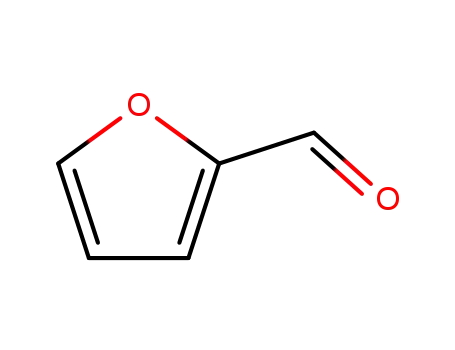
furfural

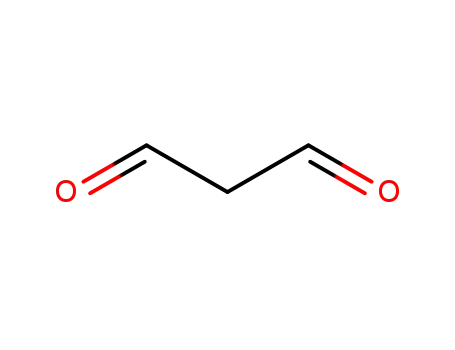
Malondialdehyde

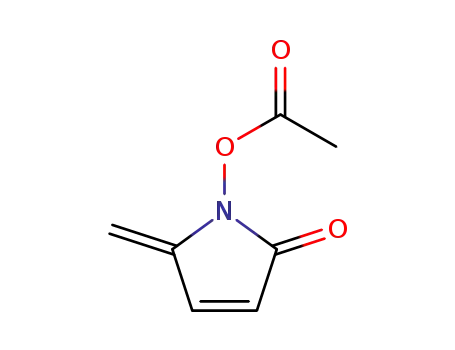
N-oxycarbonylmethyl-5-methylene-Δ3-pyrrolin-2-one

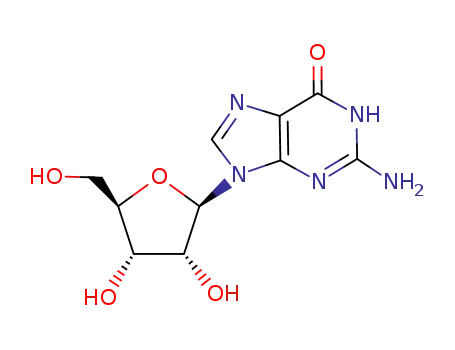
G

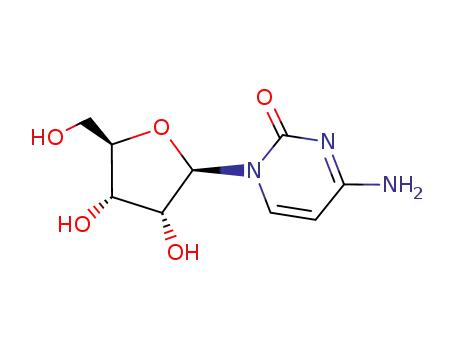
CYTIDINE

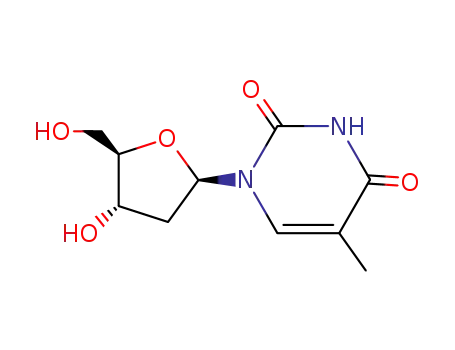
thymidine

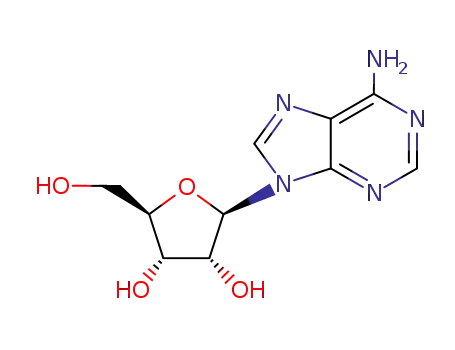
adenosine
| Conditions | Yield |
|---|---|
|
With carbonatopentamminecobalt(III) nitrate hemihydrate; In aq. phosphate buffer; at 20 ℃; for 0.133333h; pH=6.9; UV-irradiation;
|
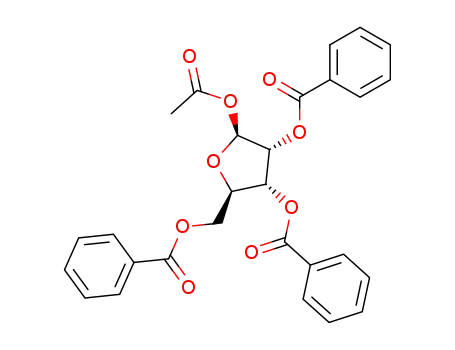
1-O-acetyl-2,3,5-tri-O-benzoyl-β-D-ribofuranose
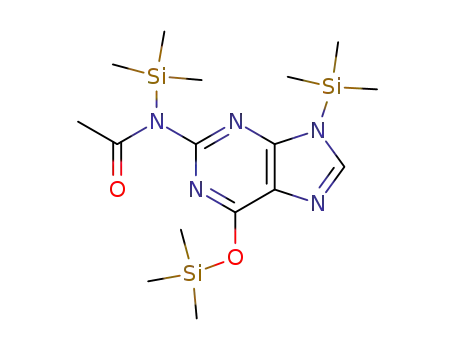
(Ac)G(TMS)2
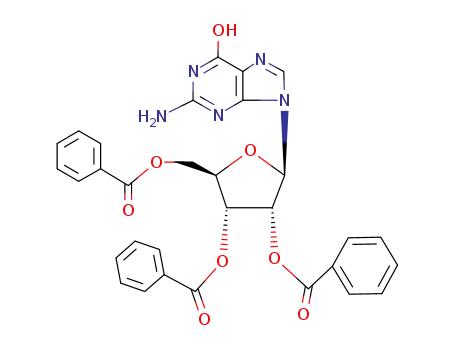
O2',O3',O5'-tribenzoyl-guanosine
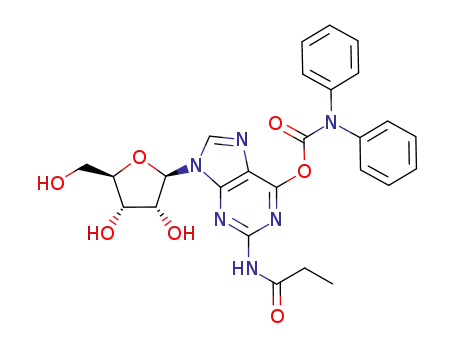
O6-(diphenylcarbamoyl)-N2-propionylguanosine
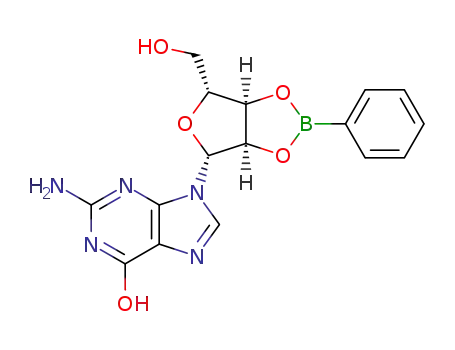
O2',O3'-phenylboranediyl-guanosine
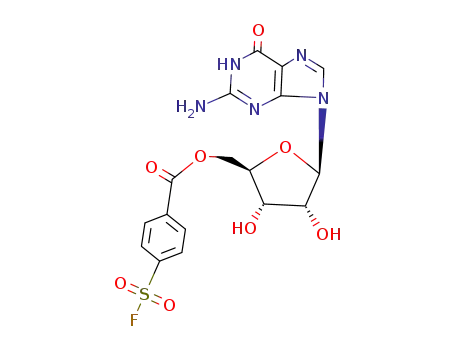
5'-
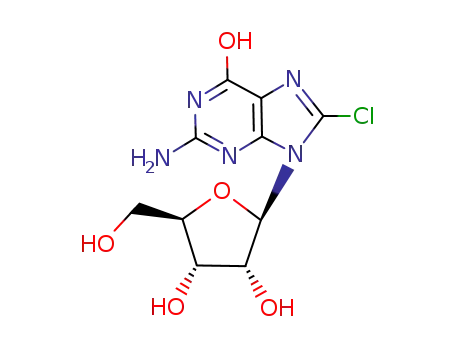
8-chloroguanosine
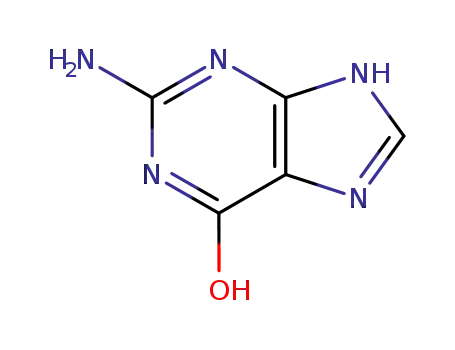
2-amino-6-hydroxypurine