- +86 185 8909 5435
- sales@senovaph.com
Your Location:Home >Products >179688-53-0

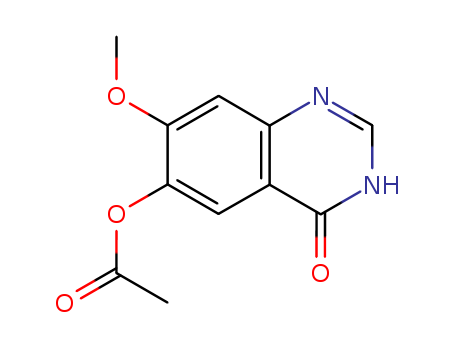
pd_meltingpoint:293 °C
Purity:99%
InChI:InChI=1/C11H10N2O4/c1-6(14)17-10-3-7-8(4-9(10)16-2)12-5-13-11(7)15/h3-5H,1-2H3,(H,12,13,15)
Multi-target, especially dual-target, dr...
Compounds and methods for their preparat...
The invention discloses a polysubstitute...
Accurate prediction of absolute protein-...
acetic anhydride

3H-6,7-dimethoxyquinazolin-4-one

6-acetoxy-7-methoxy-3,4-dihydroquinazolin-4-one
| Conditions | Yield |
|---|---|
|
3H-6,7-dimethoxyquinazolin-4-one; With methanesulfonic acid; DL-methionine; at 120 ℃; for 24h;
acetic anhydride; With pyridine; at 100 ℃; for 22h;
|
87% |
|
With methanesulfonic acid; DL-methionine; Multistep reaction; 1.) 100 deg C, 3 h, 2.) Py;
|
acetic anhydride

6-hydroxy-7-methoxyquinazolin-4(3H)-one

6-acetoxy-7-methoxy-3,4-dihydroquinazolin-4-one
| Conditions | Yield |
|---|---|
|
With pyridine; at 100 ℃; for 4h;
|
99% |
|
With pyridine; at 100 ℃; for 2h;
|
99% |
|
With pyridine; at 100 ℃; for 2h;
|
96.6% |
|
With pyridine; In N,N-dimethyl-formamide; at 40 - 45 ℃; for 1h;
|
96% |
|
With pyridine; at 100 ℃; for 4h;
|
93% |
|
With pyridine; at 100 ℃; for 4h;
|
93% |
|
In pyridine; at 116 ℃; for 3h;
|
82% |
|
at 120 - 125 ℃; for 3h;
|
75% |
|
With pyridine; at 100 ℃; for 6h;
|
74% |
|
In pyridine; Inert atmosphere;
|
62% |
|
With pyridine; dmap; at 100 ℃; for 4h;
|
60% |
|
With pyridine; at 100 ℃; for 4h;
|
53% |
|
With pyridine; at 20 - 100 ℃; for 3h;
|
53% |
|
With pyridine; at 100 ℃; for 3h;
|
53% |
|
With pyridine; at 100 ℃;
|
51% |
|
With pyridine; at 100 ℃; for 6h;
|
51% |
|
With pyridine; for 3h; Heating / reflux;
|
50% |
|
With pyridine; at 100 ℃; for 2h;
|
41.3% |
|
With pyridine; dmap; at 100 ℃; for 6h; Inert atmosphere;
|
40% |
|
With pyridine;
|
|
|
With pyridine; dmap; at 100 ℃; for 6h;
|
|
|
In pyridine; for 3h; Heating / reflux;
|
|
|
With pyridine; for 3h; Heating / reflux;
|
|
|
With pyridine; at 100 ℃; for 4h;
|
|
|
With pyridine; dmap; at 0 - 20 ℃; for 12h; Inert atmosphere;
|
|
|
With pyridine; dmap; at 100 ℃;
|
|
|
With dmap; In pyridine;
|
|
|
With pyridine; dmap; at 0 - 20 ℃; for 12h;
|
|
|
With pyridine; dmap; at 0 - 20 ℃; for 12h;
|
|
|
With pyridine; at 20 ℃;
|
|
|
With pyridine; for 3h; Heating / reflux;
|
|
|
acetic anhydride; 6-hydroxy-7-methoxyquinazolin-4(3H)-one; With pyridine; at 100 ℃; for 1h;
With dmap; for 5h;
|
|
|
With pyridine; In N,N-dimethyl-formamide; at 40 - 45 ℃; for 1h;
|
117 g |
|
With pyridine; Reflux;
|
acetic anhydride
3H-6,7-dimethoxyquinazolin-4-one
6-hydroxy-7-methoxyquinazolin-4(3H)-one
methyl 2-amino-4,5-dimethoxybenzoate
4-chloro-7-methoxyquinazolin-6-yl acetate hydrochloride salt
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline
gefitinib
6,7-dihydroxyquinazolin-4(3H)-one