- +86 185 8909 5435
- sales@senovaph.com
Your Location:Home >Products >Organic Chemistry >127852-28-2

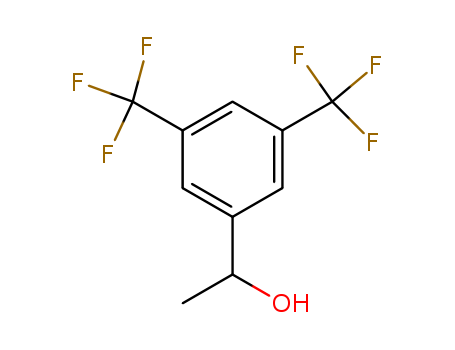
pd_meltingpoint:53-58℃
Purity:99%
|
Preparation |
At present, the methods for preparing optically pure (R)-[3,5-bis (trifluoromethyl) phenyl] ethanol include chemical methods and biological methods. Chemical synthesis requires the use of expensive transition metal Ru and other chemical catalysts, complicated steps, harsh reaction conditions, high energy consumption, large pollution, and low yield. Biological law is the use of free enzymes or whole cells for catalytic preparation. It has the characteristics of mild reaction conditions, high catalytic efficiency, and strong specificity. |
|
General Description |
(R)-1-[3,5-Bis(trifluoromethyl)phenyl]ethanol is a key intermediate for the synthesis of aprepitant, a potent human neurokinin-1 (NK-1) receptor. |
InChI:InChI=1/C10H8F6O/c1-5(17)6-2-7(9(11,12)13)4-8(3-6)10(14,15)16/h2-5,17H,1H3
A novel efficient asymmetric hydrogenati...
A simple and convergent approach to enan...
Enzyme-catalyzed efficient synthesis of ...
A total of 82 fungal isolates were scree...
We investigated the asymmetric bioreduct...
Two complementary approaches for the ena...
A strain of Chryseobacterium sp. CA49 wa...
(R)-3,5-Bistrifluoromethylphenyl ethanol...
The synthesis of (S)-3,5-bistrifluoromet...
Reported is the synthesis of a number of...
This study was designed to determine whe...
Leifsonia xyli HS0904 can stereoselectiv...
Dynamic kinetic resolution (DKR) of vari...
A novel PNN ligand bearing an orthopheny...
By using a copper transmetalation reagen...
Alcohol dehydrogenases can catalyze the ...
The development of a pilot process for t...
The enantioselective hydrogenation of ri...
A chiral cobalt pincer complex, when com...
Novel chiral cobalt complex a containing...
I-interacting ligands of the diphosphino...
Asymmetric catalytic reactions are among...
vinyl acetate

1-(3,5-bis(trifluoromethyl)phenyl)ethan-1-ol

(R)-O-acetyl-1-(3,5-bis(trifluoromethyl)phenyl)ethanol

(S)-3,5-bis(trifluoromethyl)-1-phenylethanol

(R)-[3,5-bis(trifluoromethyl)phenyl]ethanol
| Conditions | Yield |
|---|---|
|
With Novozyme-435 lipase; In tetrahydrofuran; at 20 ℃; for 72h; Resolution of racemate; Enzymatic reaction;
|
40% 89 % ee |
|
Candida antartica lipase B; In various solvent(s); at 40 ℃; for 2h; under 67505.4 Torr;
|
Isopropenyl acetate

1-(3,5-bis(trifluoromethyl)phenyl)ethan-1-ol

(R)-O-acetyl-1-(3,5-bis(trifluoromethyl)phenyl)ethanol

(S)-3,5-bis(trifluoromethyl)-1-phenylethanol

(R)-[3,5-bis(trifluoromethyl)phenyl]ethanol
| Conditions | Yield |
|---|---|
|
With Novozyme-435 lipase; In tetrahydrofuran; at 20 ℃; for 72h; Resolution of racemate; Enzymatic reaction;
|
38% 86 % ee |
3,5-bis(trifluoromethyl)phenyl methyl ketone
3,5-bis(trifluoromethyl)phenylmagnesium bromide
acetaldehyde
1-(3,5-bis(trifluoromethyl)phenyl)ethan-1-ol
3,5-bis(trifluoromethyl)styrene
3,5-bis(trifluoromethyl)phenyl methyl ketone
(S)-3,5-bis(trifluoromethyl)-1-phenylethanol
(R)-[3,5-bis(trifluoromethyl)phenyl]ethanol