- +86 185 8909 5435
- sales@senovaph.com
Your Location:Home >Products >Pharmaceutical intermediates >149809-43-8
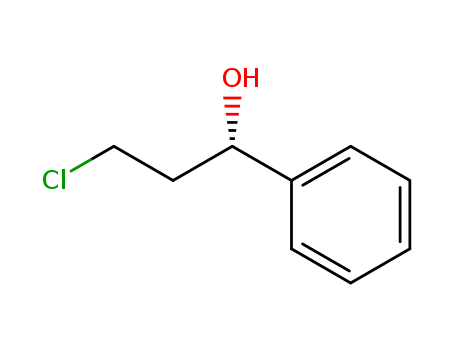

Purity:99%
|
Article |
Source |
|
Chemical Properties |
White Crystalline Solid |
|
Uses |
Posaconazole intermediate. Antifungal agent. |
InChI:InChI=1/C21H21F2N3O4S/c1-15-2-5-18(6-3-15)31(27,28)30-11-16-9-21(29-10-16,12-26-14-24-13-25-26)19-7-4-17(22)8-20(19)23/h2-8,13-14,16H,9-12H2,1H3/t16-,21-/m0/s1
The invention relates to the technical f...
The present invention relates to a compo...
The invention provides a method for prep...
The present invention relates to process...
C14H15F2N3O2*(x)ClH

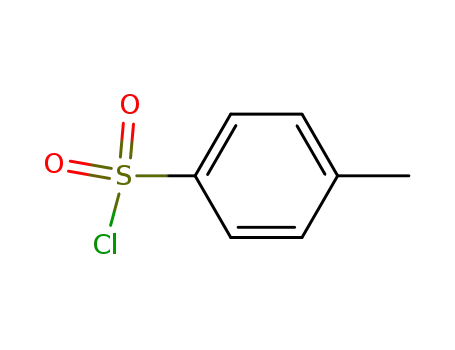
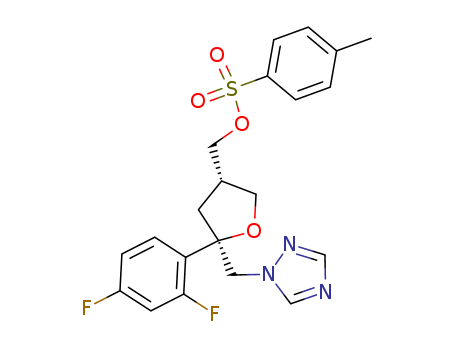
p-toluenesulfonyl chloride

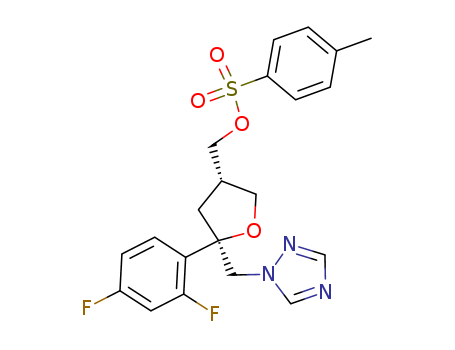
![(2R-cis)-2-(2,4-difluorophenyl)-4-[[(4-methylphenyl)sulfonyloxy]methyl]-2-[(1H-1,2,4-triazol-1-yl)methyl]tetrahydrofuran](/upload/2023/5\60e4910f-8033-40ec-920b-5f4b3aa55ffd.png)
(2R-cis)-2-(2,4-difluorophenyl)-4-[[(4-methylphenyl)sulfonyloxy]methyl]-2-[(1H-1,2,4-triazol-1-yl)methyl]tetrahydrofuran
| Conditions | Yield |
|---|---|
|
C14H15F2N3O2*(x)ClH; With triethylamine; In dichloromethane; at 12 - 22 ℃; for 1h;
p-toluenesulfonyl chloride; With dmap; In dichloromethane; at 12 - 25 ℃; for 4h;
|
94% |
C14H15F2N3O2*ClH


p-toluenesulfonyl chloride

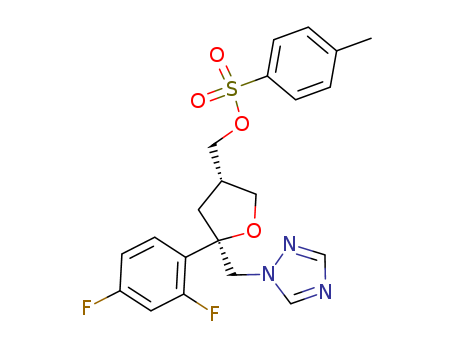
![(2R-cis)-2-(2,4-difluorophenyl)-4-[[(4-methylphenyl)sulfonyloxy]methyl]-2-[(1H-1,2,4-triazol-1-yl)methyl]tetrahydrofuran](/upload/2023/5\60e4910f-8033-40ec-920b-5f4b3aa55ffd.png)
(2R-cis)-2-(2,4-difluorophenyl)-4-[[(4-methylphenyl)sulfonyloxy]methyl]-2-[(1H-1,2,4-triazol-1-yl)methyl]tetrahydrofuran
| Conditions | Yield |
|---|---|
|
With dmap; triethylamine; In dichloromethane; at 14 - 30 ℃;
|
94% |

p-toluenesulfonyl chloride
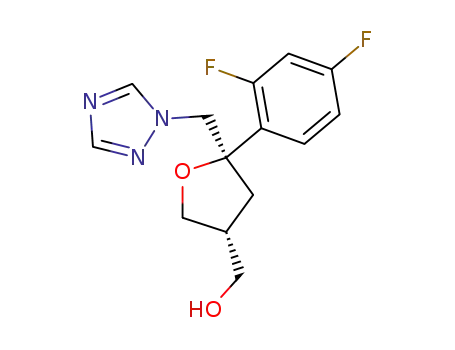
((3R,5R)-5-((1H-1,2,4-triazole-1-yl) methyl)-5-(2,4-difluorophenyl) tetrahydrofuran-3-yl)methanol
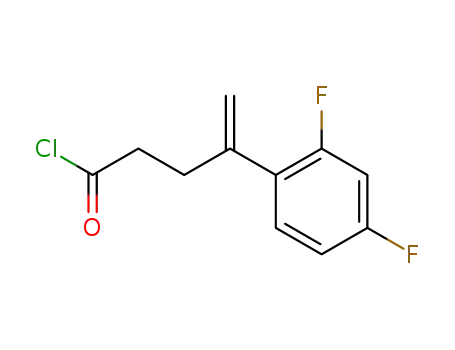
4-(2,4-Difluoro-phenyl)-pent-4-enoyl chloride
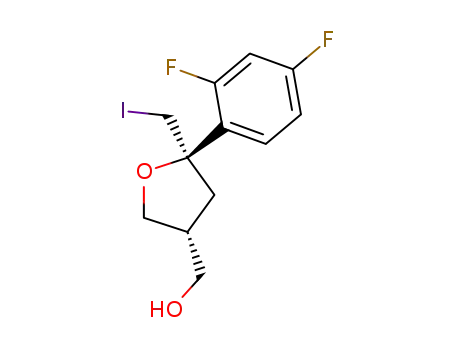
((3R,5R)-5-(2,4-difluorophenyl)-5-(iodomethyl)tetrahydrofuran-3-yl)methanol
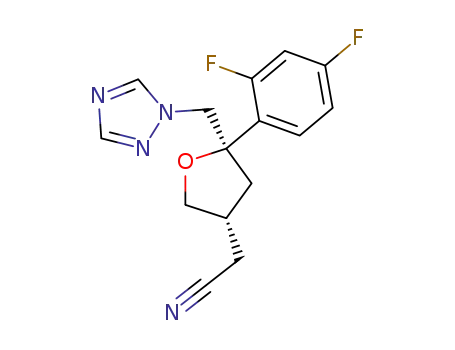
(2R-cis)-4-cyanomethyl-2-(2,4-difluorophenyl)-2-[(1H-1,2,4-triazol-1-yl)methyl]tetrahydrofuran
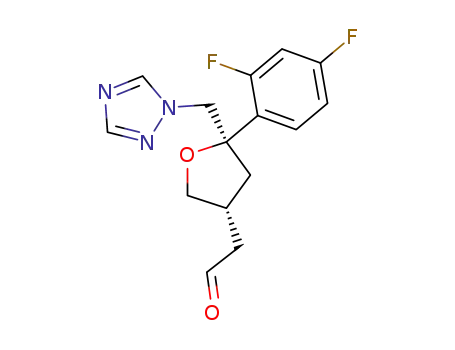
(2R-cis)-2-(2,4-difluorophenyl)-4-formylmethyl-2-[(1H-1,2,4-triazol-1-yl)methyl]tetrahydrofuran
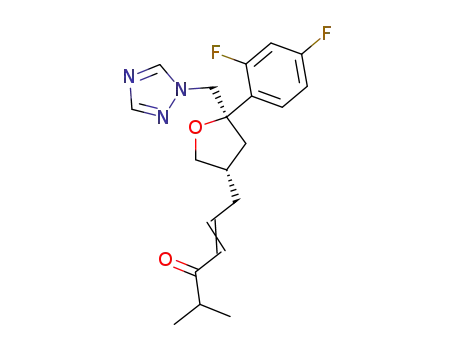
(2R-cis)-2-(2,4-difluorophenyl)-4-(5-methyl-4-oxo-2-hexenyl)-2-[(1H-1,2,4-triazol-1-yl)methyl]tetrahydrofuran
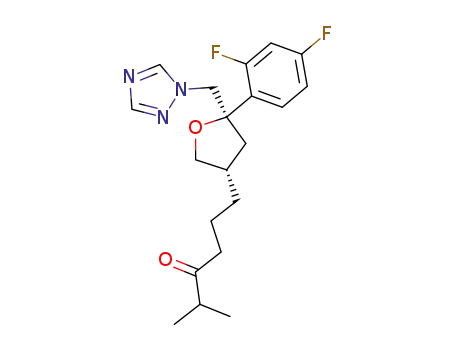
(2R-cis)-2-(2,4-difluorophenyl)-4-(5-methyl-4-oxohexyl)-2-[(1H-1,2,4-triazol-1-yl)methyl]tetrahydrofuran